*BD reserves the right to terminate the Program at any time.
**Please note, signed Crosser iQ™ Ultrasonic CTO Device Purchase Agreement terms and conditions apply.
1 26/30 devices successfully crossed an 8 cm synthetic lesion model made of 10 mm of formula 8 on each end (replicating calcified end caps (no specified cap shape)) and 60 mm of formula 4 (replicating the fibrous middle of a CTO). Crossing success was defined as passing through the entire simulated lesion within 5 minutes or less of device activation. Device activation time ranged from 12 to 290 seconds. Device activation time was measured from the time of device engagement of the simulated lesion until the device crossed through the distal cap of the simulated lesion or until the device life expired and device activation stopped.
238/40 devices engaged an Ultracal30 gypsum convex cap stone successfully. Engagement success was defined as ability of the device to engage the simulated cap within 30 seconds of device activation.
Bench data on file, BD, Tempe, AZ. Bench data may not necessarily correlate to clinical performance. Different test methods may yield different results. Actual user experience may vary.
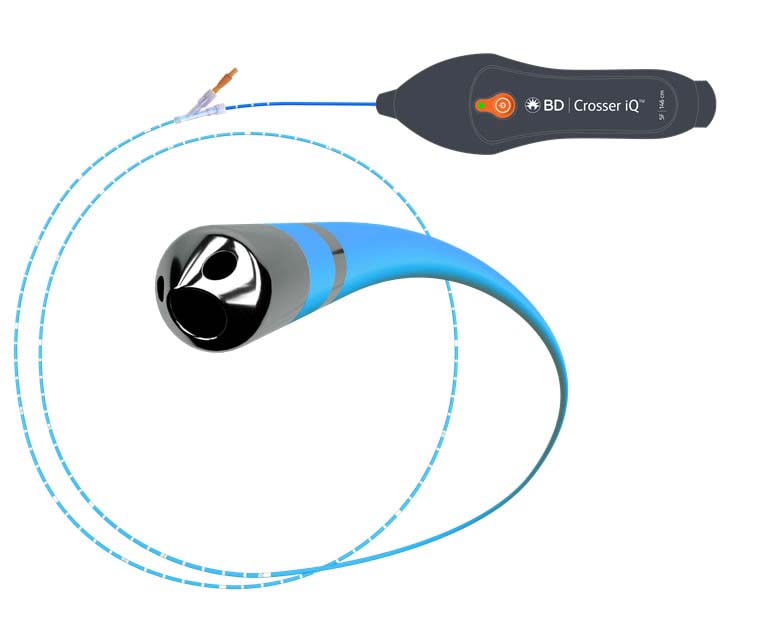
The BD Recanalization System (Console and Footswitch) and the Crosser iQ™ Ultrasonic CTO Device are indicated to facilitate the intra-luminal placement of conventional guidewires beyond peripheral artery chronic total occlusions. The Crosser iQ™ Ultrasonic CTO Device is contraindicated for use in the carotid arteries.
Potential Adverse Events: Bleeding which may require transfusion or surgical intervention· Puncture site hematoma, pain and tenderness · Hemorrhage · Embolism · Vessel perforation/dissection · Guidewire entrapment and/or fracture · Hypertension/Hypotension · Infection or fever · Allergic reaction · Pseudoaneurysm or fistula Aneurysm · Acute reclosure · Thrombosis · Ischemic events · Distal embolization · Excessive contrast load resulting in renal insufficiency or failure · Excessive exposure to radiation · Stroke/CVA · Restenosis · Repeat catheterization/angioplasty · Peripheral artery bypass · Amputation · Death · Other bleeding complications at access site.