
Let’s achieve more for your patients, every single day
We are patient-first innovators. Every advancement we have brought to market as Bard® and now. BD, has been centered on supporting health care professionals in improving patient health and care.
Explore the BD® Acute Urology approach to disease states
Catheter-associated urinary tract infections (CAUTIs) are infections that occur in the urinary tract due to the use of a catheter.1 These infections are a common type of healthcare-associated infection (HAI) and can lead to serious complications if not properly managed.2 In 2009, the CDC introduced Guidelines for the Prevention of Catheter-Associated Urinary Tract Infections to help clinicians minimize the risk of CAUTIs in their patients.3 *
Becton, Dickinson and Company (BD®) Acute Urology product portfolio is designed for clinicians aiming to comply with these guidelines.
The CDC guidelines emphasize minimizing the risk of CAUTIs by recommending:
BD offers products in each of these categories, helping health care providers align with the CDC’s recommendations for effective catheter management.* Additionally, BD offers the Bardex™ I.C. Foley catheter with Bactiguard® coating, which has been shown to lower the occurrence of CAUTI in multiple clinical studies.4,5,6,7
*The CDC does not endorse specific brands of products.
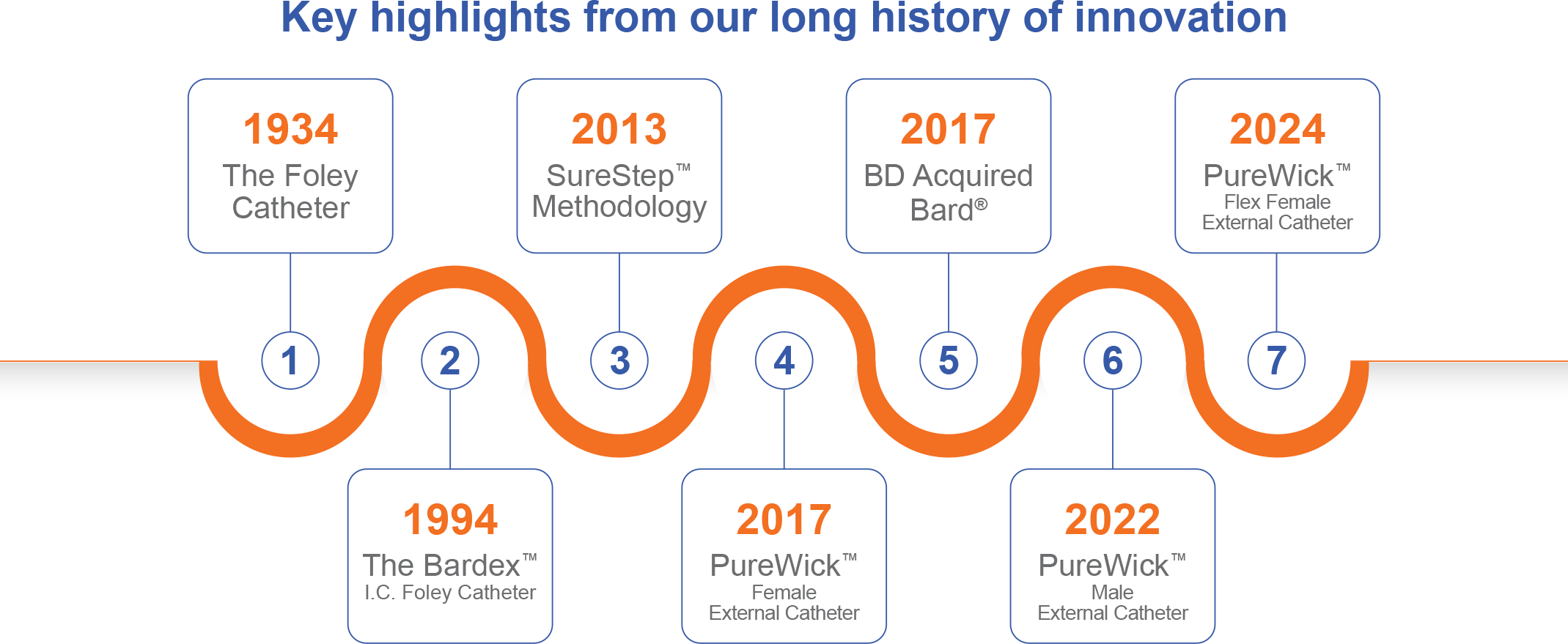
Past, Present, Future: A commitment to excellence
BD® Acute Urology’s commitment to excellence is informed by the past and drives the future. We are proud of our history providing forward-thinking products and innovative technologies that support our health care professional partners and their patients. This showcases only a small portion of the numerous innovations designed to advance excellence in patient care.
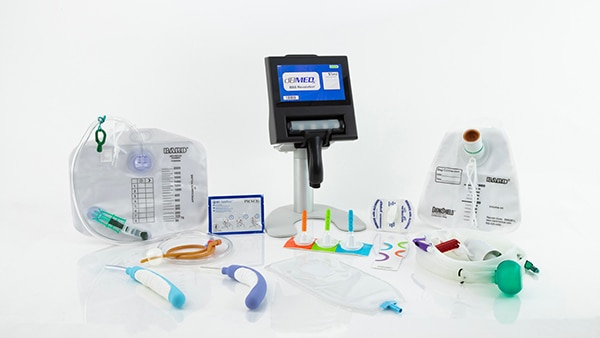
Innovations that address challenges across the continuum of care
BD® Acute Urology employs a patient-centric approach to industry advancement and innovation. The result is a breadth of products that address varying needs across patient populations and along multiple touchpoints of the hospital journey, including the journey home.
Indwelling Foley
External Catheter Options
Bladder Retention
Urinary Drainage Accessories
Urine Output System
Fecal Management
BD® Clinical Connect
Product Training and Education Program

Working toward the best patient experience possible
At the heart of BD® Acute Urology are our Clinical Managers. This group consists of licensed RNs who inform our product training and education for health care professionals. Their expertise drives advancements and innovation for the benefit of our patients.
Through the BD® Clinical Connect program, our Clinical Managers partner with health care professionals to help continuously provide the best patient experience possible.
BD® Clinical Connect provides access to the right training at the right time for the right application. As partners, let’s achieve more for your patients, every single day.
- Centers for Disease Control and Prevention. Catheter-associated Urinary Tract Infection (CAUTI) basics. https://www.cdc.gov/uti/about/cauti-basics.html. Accessed November 26, 2024.
- WebMD. What to Know About CAUTIs. https://www.webmd.com/a-to-z-guides/what-to-know-cautis. Accessed November 26, 2024.
- Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA; Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for Prevention of Catheter-Associated Urinary Tract Infections 2009. Centers for Disease Control and Prevention. Updated February 15, 2017. Available from: https://www.cdc.gov/infectioncontrol/guidelines/cauti/. Accessed November 26, 2024.
- Kai-Larsen Y, Grass S, Mody B, et al. Foley catheter with noble metal alloy coating for preventing catheter-associated urinary tract infections: a large, multi-center clinical trial. Antimicrob Resist Infect Control. 2021;10(1):40.
- Hidalgo Fabrellas et al. Enferm Intensiva. 2015; 26(2):54–62.
- Lederer JW, Jarvis WR, Thomas L, Ritter J. Multicenter cohort study to assess the impact of a silver-alloy and hydrogel-coated urinary catheter on symptomatic catheter-associated urinary tract infections. J Wound Ostomy Continence Nurs. 2014;41(5):473-480.
- Newton T, Still JM, Law E. A comparison of the effect of early insertion of standard latex and silver-impregnated latex Foley catheters on urinary tract infections in burn patients. Infect Control Hosp Epidemiol. 2002;23(4):217-218.
Foley Catheters – Foley catheters are intended for use in the drainage and/or collection and/or measurement of urine. Cautions: Latex Foley catheters contain natural rubber latex which may cause allergic reactions. Federal (U.S.A.) law restricts these devices to sale by or on the order of a physician.
Warnings: On catheter, do not use ointments or lubricants having a petrolatum base. They will damage the catheter and may cause balloon to burst. After use, this product may be a potential biohazard. Handle and dispose of in accordance with accepted medical practices and applicable local, state and federal laws and regulations. Visually inspect the product for any imperfections or surface deterioration prior to use. If package is opened or if any imperfection or surface deterioration is observed, do not use.
Please consult product labels, inserts and instructions for use for any indications, contraindications, hazards, warnings, cautions and directions for use.
BD, the BD Logo, Bard, PureWick, SureStep, Bardex, StatLock, Sensica, DigniShield and Permalene are trademarks of Becton, Dickinson and Company or its affiliates. Bactiguard®* is a registered trademark of Bactiguard AB. *The Foley catheters included in the Bardex™ I.C. System contain Bactiguard®* silver alloy coating which is licensed from BactiGuard AB.
