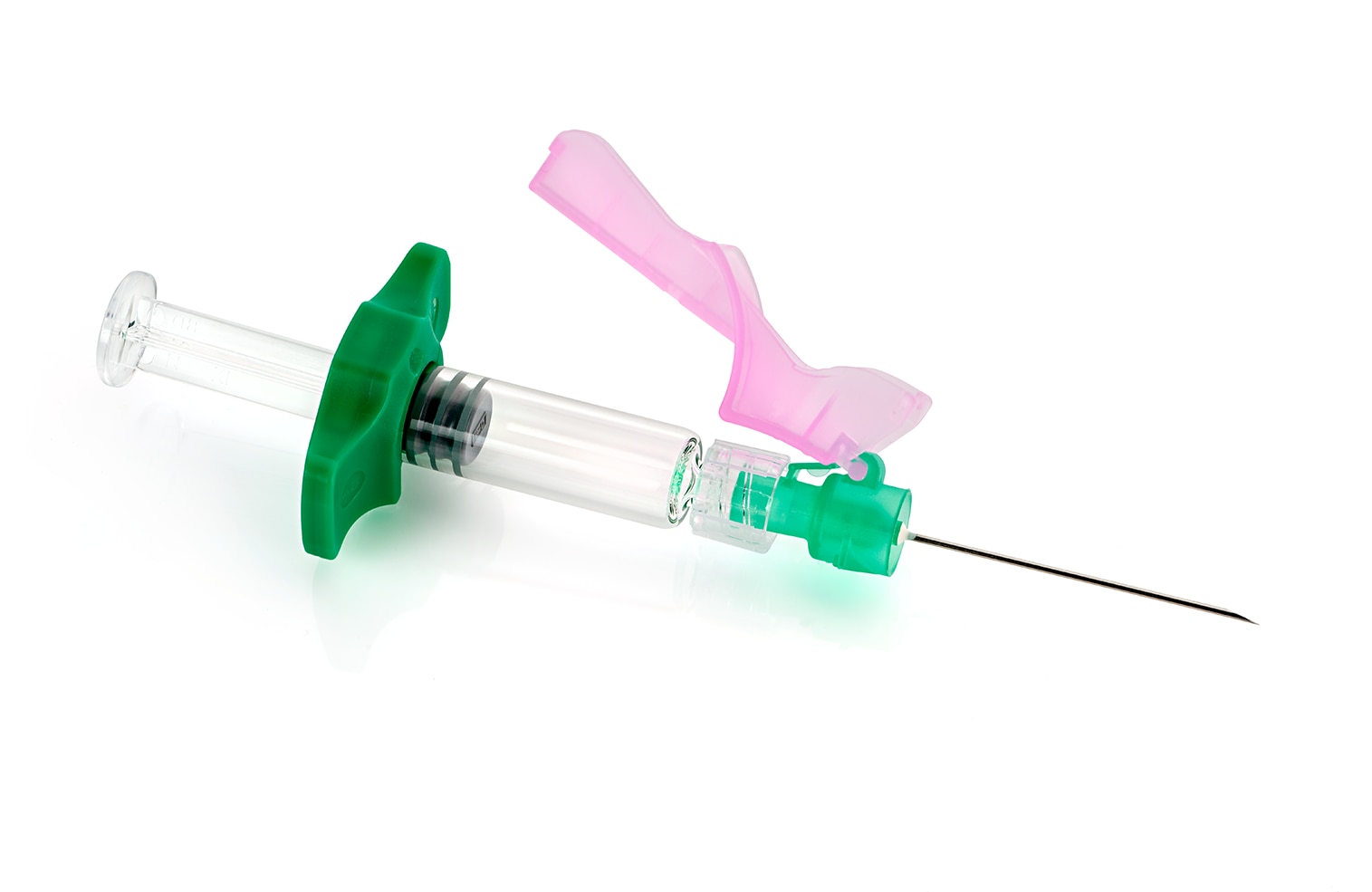
BD Effivax™ Glass Prefillable Syringe
BD tools for vaccine combination product developers

- Overview
- Expertise
- Products & Accessories
- Resources
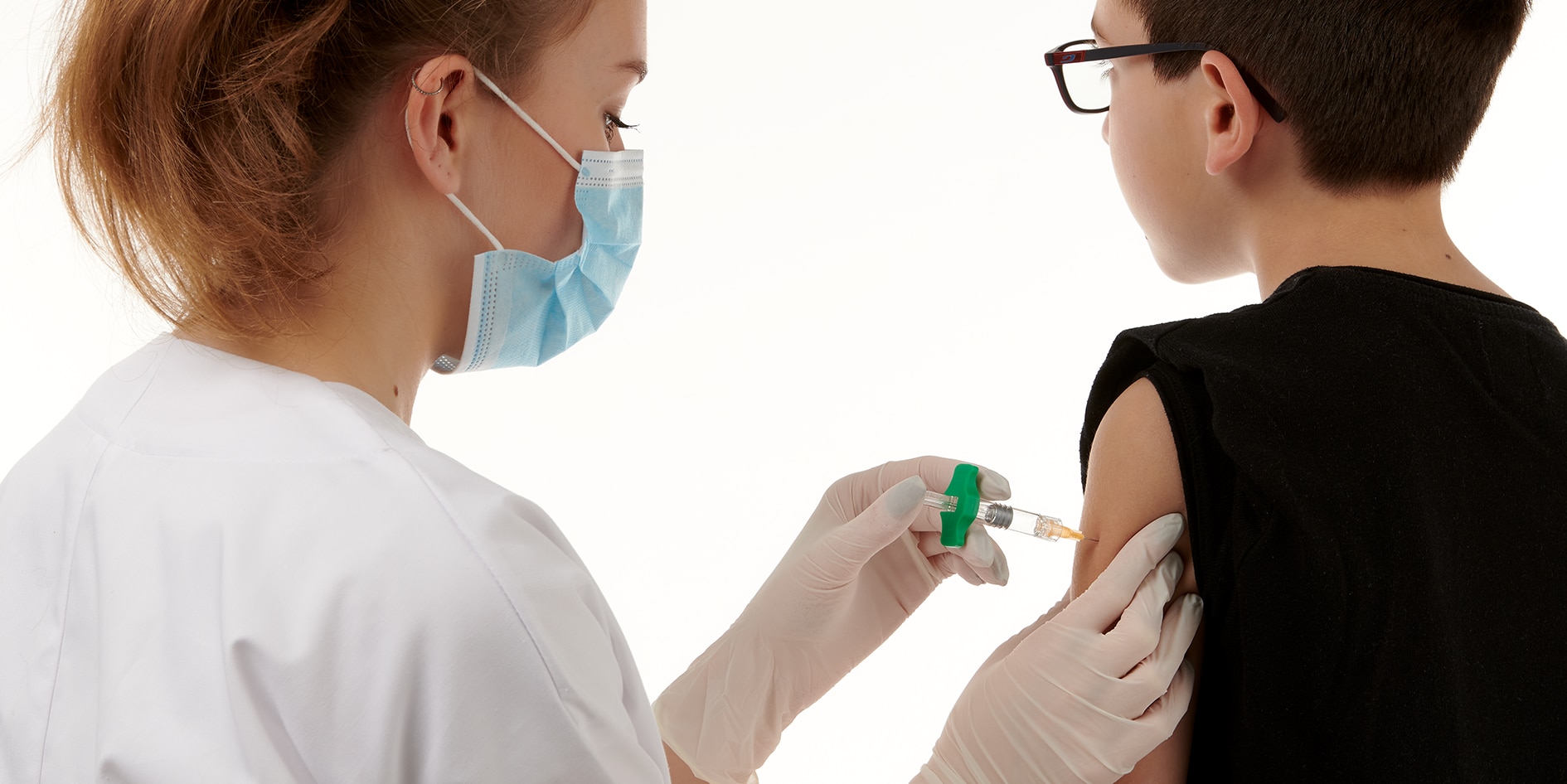
BD Effivax™ Glass Prefillable Syringe is a single use Sterile Clean and Ready to Fill syringe (BD SCF™), designed, when combined with an appropriate needle and injection technique, for manual intramuscular or subcutaneous injections of pharmaceutical drugs.1 Featuring a new vaccine prefillable syringe (PFS) barrel, and a step forward in luer lock PFS performance, the BD Effivax™ Glass Prefillable Syringe is designed to improve Total Cost of Ownership (TCO).2
BD Effivax™ Glass Prefillable Syringe Features
- Luer Lock Plastic Rigid Tip Cap (PRTC)
- Stopper with BD VisioguardTM option, a stopper inspection technology**
- Glass container and flange specifications improving operational efficiency*1
- ≥80N flange resistance2
- Color options available for customization
BD Effivax™ Glass Prefillable Syringe Benefits
- New PFS (prefillable syringe) barrel, carrier for future innovation.
- A step forward in luer lock PFS performance as compared to previous generation BD vaccine PFS*:
- Overall integrity improved due to the reduction of cracks and contamination specifications.1
- Stricter glass particles specification.1
- 100%-dimensional controls (ISO11040-4).
- Stricter visible cosmetic defects specifications.1
- Control plan harmonized across manufacturing sites for product consistency.3
BD Effivax™ Glass Prefillable Syringe Benefits
- Designed to improve Total Cost of Ownership (TCO).2
- Filling and packing optimized, consequently minimizing line stops.
- Batch performance documentation (CoA).
- Broad range of value-added services.***
- Regulatory expertise in combination product.
- Functional tests in c-GMP compliant labs.
- E&L studies with toxicological assessment.
- Multi-sourcing network options for business continuity:
- 2 plants : in Europe and in North America.
*compared with BD Hypak™ for Vaccines;
**BD Visioguard™: features 100% automated visual inspection to identify defects and support operational excellence;
*** non exhaustive list;
† BD implemented control plans to reduce BD Effivax™ product variability and optimize filling and packing, consequently improving operational efficiency by minimizing line stops.
CoA : Certificate of Analysis; c-GMP: Current Good Manufacturing Practices; E&L: Extractables and Leachables
- BD internal reference, Customer Quality Specification BD Effivax™ SC000243, BD Hypak(™) for Vaccine SC000110, BD Medical – Pharmaceutical Systems Le Pont de Claix, France
- BD internal analysis, TCO Model, BD Medical – Pharmaceutical Systems Le Pont de Claix, France
- BD internal reference, Global Product Manufacturing Specification 442.GPMS.060, BD Medical – Pharmaceutical Systems Le Pont de Claix, France
- BD sales analysis [internal analysis]. Pont-de-Claix, FR: Becton, Dickinson and Company; 2022
- Vaccines Market Size, Share & COVID-19 Impact Analysis, Market Research Report, https://www.fortunebusinessinsights.com/industry-reports/vaccines-market-101769, February 2022
- BD sales analysis [internal analysis]. Pont-de-Claix, FR: Becton, Dickinson and Company; 2021
BD Expertise in the BD Effivax™ Glass Prefillable Syringe
- Ordered by a major pharmaceutical manufacturer.4
- BD Effivax™ Glass Prefillable Syringe is built on years of BD expertise partnering with leading pharmaceutical companies.5,6
- BD is uniquely positioned with high volume production capacity delivering hundreds of millions of PFS for vaccines each year.6
*compared with BD Hypak™ for Vaccines;
**BD Visioguard™: features 100% automated visual inspection to identify defects and support operational excellence;
*** non exhaustive list;
† BD implemented control plans to reduce BD Effivax™ product variability and optimize filling and packing, consequently improving operational efficiency by minimizing line stops.
CoA : Certificate of Analysis; c-GMP: Current Good Manufacturing Practices; E&L: Extractables and Leachables
- BD internal reference, Customer Quality Specification BD Effivax™ SC000243, BD Hypak(™) for Vaccine SC000110, BD Medical – Pharmaceutical Systems Le Pont de Claix, France
- BD internal analysis, TCO Model, BD Medical – Pharmaceutical Systems Le Pont de Claix, France
- BD internal reference, Global Product Manufacturing Specification 442.GPMS.060, BD Medical – Pharmaceutical Systems Le Pont de Claix, France
- BD sales analysis [internal analysis]. Pont-de-Claix, FR: Becton, Dickinson and Company; 2022
- Vaccines Market Size, Share & COVID-19 Impact Analysis, Market Research Report, https://www.fortunebusinessinsights.com/industry-reports/vaccines-market-101769, February 2022
- BD sales analysis [internal analysis]. Pont-de-Claix, FR: Becton, Dickinson and Company; 2021
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.