RELATED PRODUCTS NOT AVAILABLE
true

- Overview
- Experience
- Availability & Support
- Products & Accessories
- Resources
BD Evolve™ On-body Injector is a product in development; some statements made are forward-looking that are subject to regulatory approvals and a variety of risks and uncertainties.
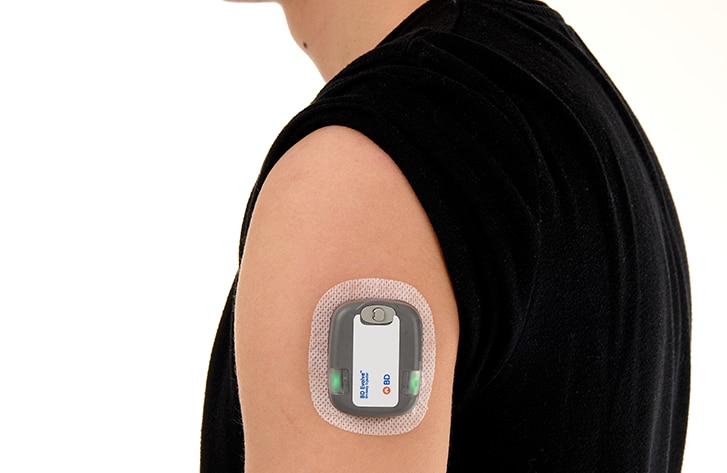
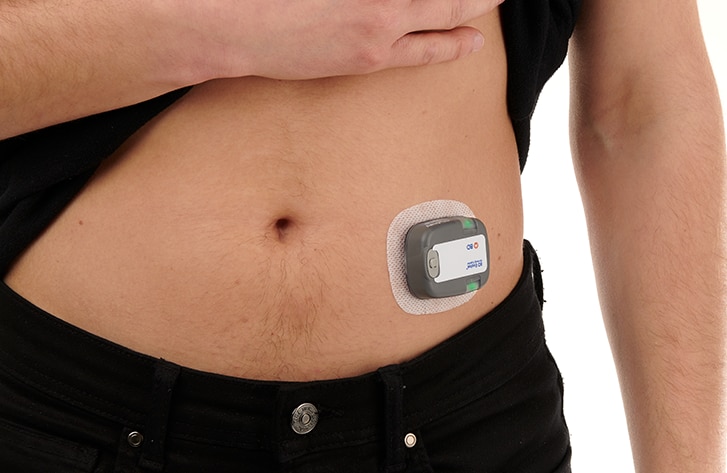
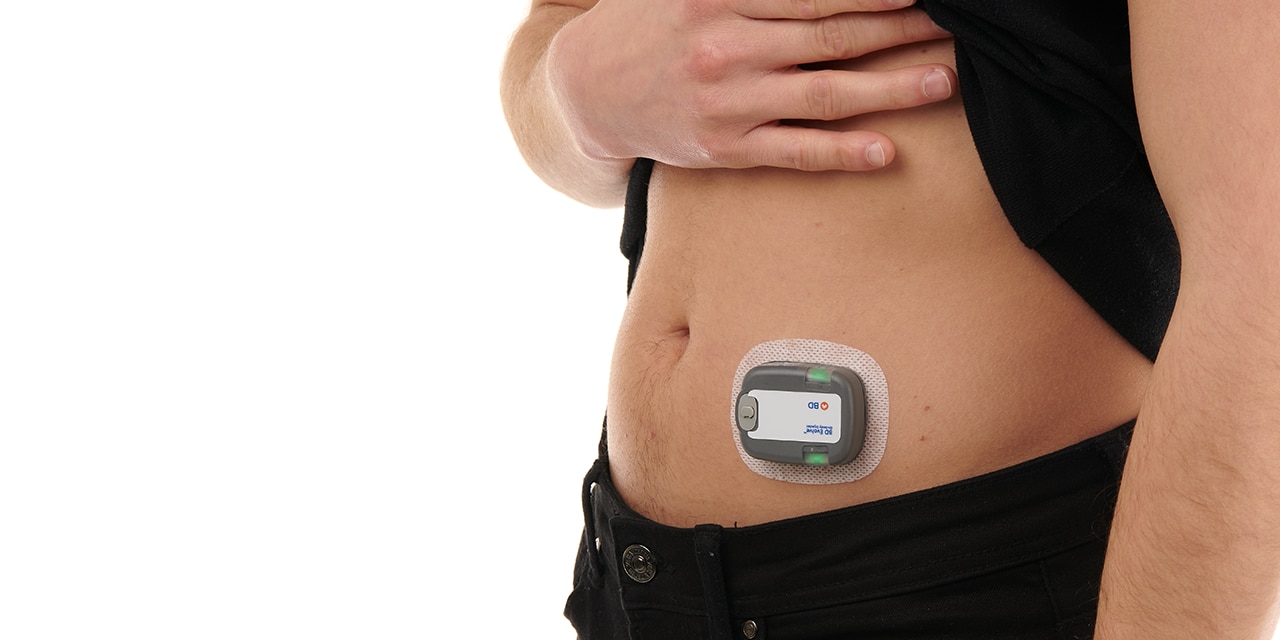
For the delivery of complex biologics, the BD Evolve™ On-body Injector is intended to offer flexibility to serve design space requirements for the continuous, episodic or delayed delivery of subcutaneous volumes1 of up to 3mL, for up to 3 days. Designed to be programmable and for connected capabilities2, the BD Evolve™ On-body Injector is intended to allow convenient medication delivery outside the acute care setting. With this solution, BD aims to address 4 primary issues within Pharma and Healthcare:
- Complex and variable treatment regimens that may impact patient acceptance3
- Cost reduction while improving patient experience and outcomes3,4
- Differentiation of drug therapies within an increasingly competitive market
- Improvement of data on patient compliance5
BD EvolveTM On-body Injector Features
- Audible feedback
- User triggered catheter insertion
- Fill volume indicator
- Subcutaneous delivery through soft catheter
- High intensity illuminated actuation buttons
BD EvolveTM On-body Injector intended benefits
- With BD EvolveTM on-body injector, BD offers flexibility to serve design space requirements for continuous, episodic, or delayed subcutaneous delivery1 of volumes up to 3 mL for up to 3-day wear.
- The system is designed to be programmable, with connected capabilities,2 allowing for convenient medication delivery outside of acute care settings.
BD EvolveTM On-body Injector is a device component intended for drug-device combination products and not subject to FDA 510(k) clearance.
- BD EvolveTM Capabilities ver1.1
- Internal Data. BD EvolveTM Preclinical Studies 20201118 BDTI
- Hong Wen, Huijeong Jung, Xuhong Li. Drug Delivery Approaches in Addressing Clinical Pharmacology-Related Issues: Opportunities and Challenges. AAPS Journal, Vol 17, No 6, Nov. 2015. DOI: 10.1.1208/s12248-051-9814-9
- Delgado Sanchez O. Comparative Cost Analysis of Intravenous and Subcutaneous Administration of Rituximab in Lymphoma Patients. Clinicalecon Outcomes Res 2019 Nov 18; 11:695-701
- Bart J.F. van den Bemt, Lynda Gettings, Barbara Domanska, Richard Burggraber, Irina Mountian and Lars E. Kristensen. A portfolio of biologic self-injection devices in rheumatology; how patient involvement in device design can improve treatment experience. Drug Delivery 2019;26(1):384-392.
- Easy to use: activate, fill & apply6
- 9 pre-clinical studies conducted to evaluate injection performance and to inform device design2
- 5 human factor studies conducted to inform device design6
- Automated manufacturing line has been established to address the needs of an integrated medical device
BD Evolve™ On-body Injector is a product in development; some statements made are forward-looking that are subject to regulatory approvals and a variety of risks and uncertainties.
BD Evolve™ On-body Injector is a device component intended for drug-device combination products and not subject to FDA 510(k) clearance.
- BD Evolve™ Capabilities ver1.1
- Internal Data. BD Evolve™ Preclinical Studies 20201118 BDTI
- Hong Wen, Huijeong Jung, Xuhong Li. Drug Delivery Approaches in Addressing Clinical PharmacologyRelated Issues: Opportunities and Challenges. AAPS Journal, Vol 17, No 6, Nov. 2015. DOI: 10.1.1208/s12248051-9814-9
- Delgado Sanchez O. Comparative Cost Analysis of Intravenous and Subcutaneous Administration of Rituximab in Lymphoma Patients. Clinicalecon Outcomes Res 2019 Nov 18; 11:695-701
- Bart J.F. van den Bemt, Lynda Gettings, Barbara Domanska, Richard Burggraber, Irina Mountian and Lars E. Kristensen A portfolio of biologic self-injection devices in rheumatology; how patient involvement in device design can improve treatment experience Drug Delivery 2019;26(1):384-392
- BD Evolve™ List of Studies v1.0
- Available to support pharma clinical development
- Samples available upon request
- Data available to support combination product
BD EvolveTM On-Body Injector is a product in development; some statements made are forward-looking that are subject to regulatory approvals and a variety of risks and uncertainties.
BD EvolveTM On-body Injector is a device component intended for drug-device combination products and not subject to FDA 510(k) clearance.
Literature
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.
Learn more
true
true