BD Hylok™ Glass Prefillable Syringe for Intravenous Drugs
Integrate the BD Hylok™ Glass Prefillable Syringe into your generic drug launch or Life Cycle Management strategy.

- Overview
- Products & Accessories
- Resources
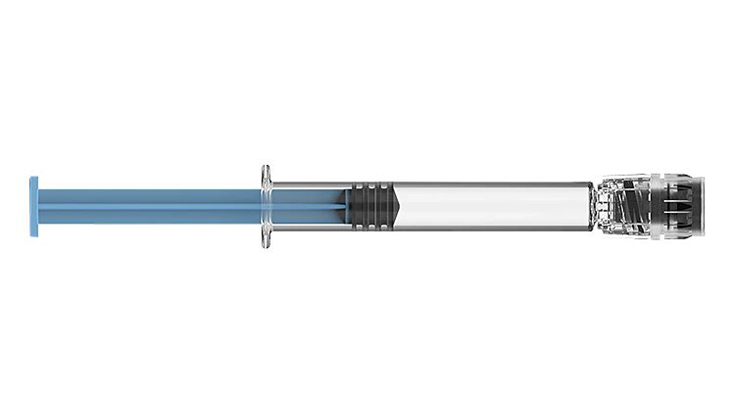
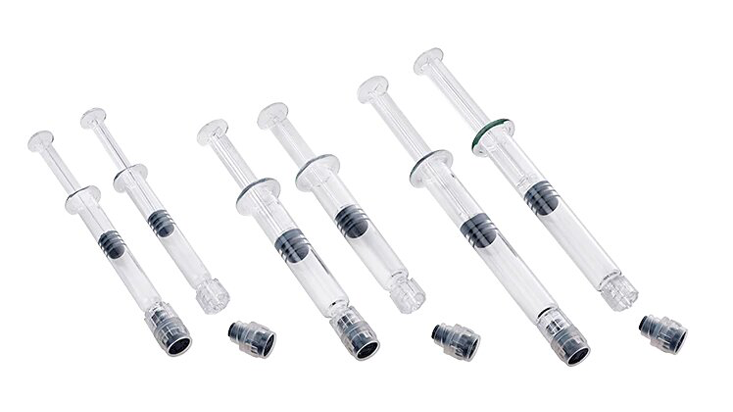
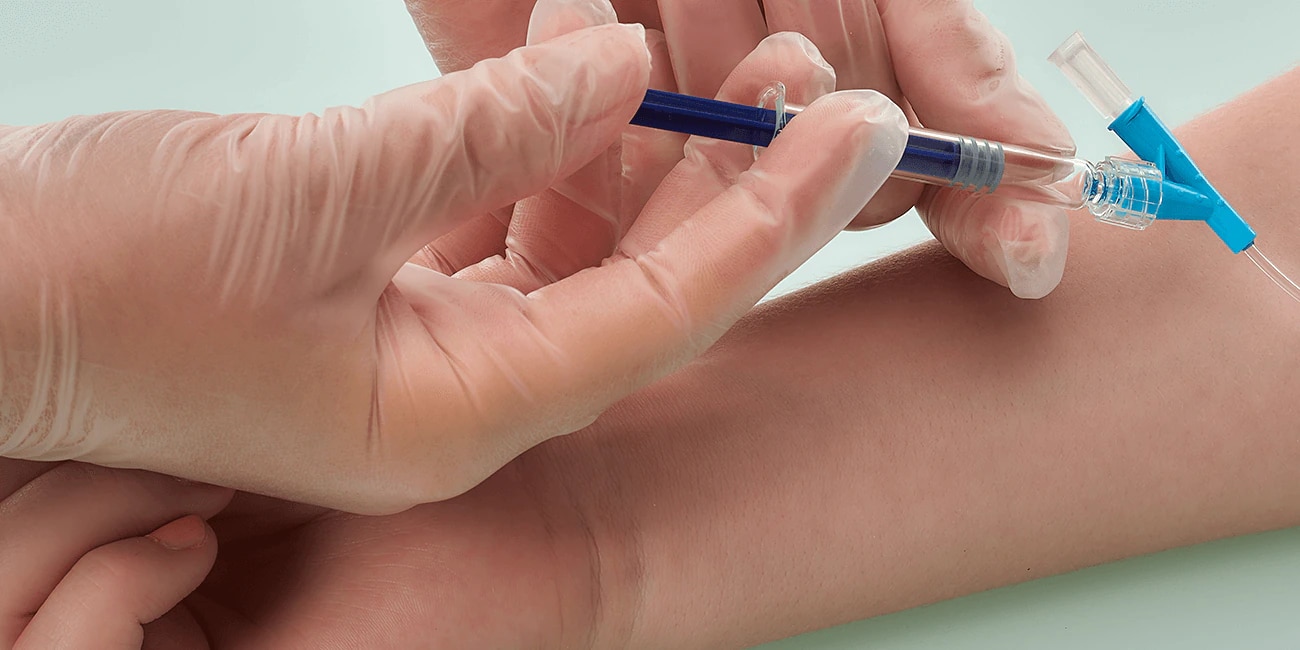
BD Hylok™ Glass Prefillable Syringe for IV Injection
A luer syringe for the administration of IV drugs. It offers customers the advantages of glass containers (e.g., medication stability) with a robust
luer connection.1,2
BD Hylok™ Glass Prefillable Syringe for Intravenous Drugs
1. BD Hylok™ Design verification results [Internal Report], 2016. Pont-de-Claix, France: Becton, Dickinson and Company.
2. A simulated validation study of needle connectivity of BD Hylok™ [Human factor study, 15 nurses and 15 dermatologists], 2017. Pont-de-Claix, France: Becton, Dickinson and Company
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.