true
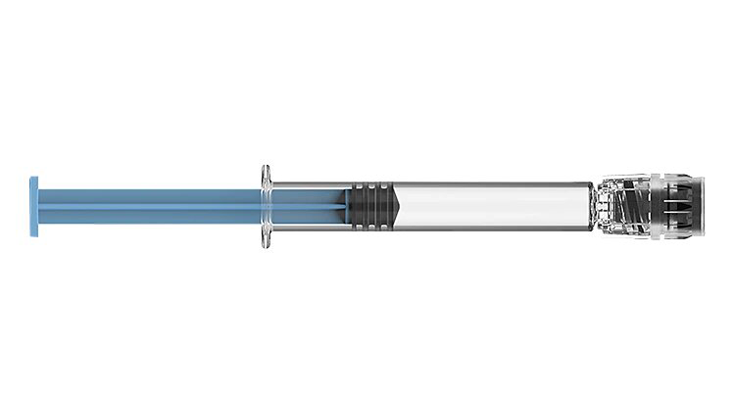
BD Hylok™ Glass Prefillable Syringe for Hyaluronic Acid
Choose innovation for hyaluronic acid


- Overview
- Products & Accessories
- Resources
BD Hylok™ Glass Prefillable Syringe for Hyaluronic Acid
A new luer syringe for the administration of viscous drugs (Hyaluronic Acid). It offers customers the advantages of glass containers (e.g., medication stability) with a robust luer connection.1,2
- Monheit GD, and Coleman KM., 2006. Hyaluronic acid fillers. Dermatol Ther. May-Jun;19(3):141-50.
- A glass syringe that optimizes needle connection and minimizes risks of leaks and maximizes [Voice of Customer research], 2017. Pont-de-Claix, France: Becton, Dickinson and Company.
Literature
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.
Learn more
true
true