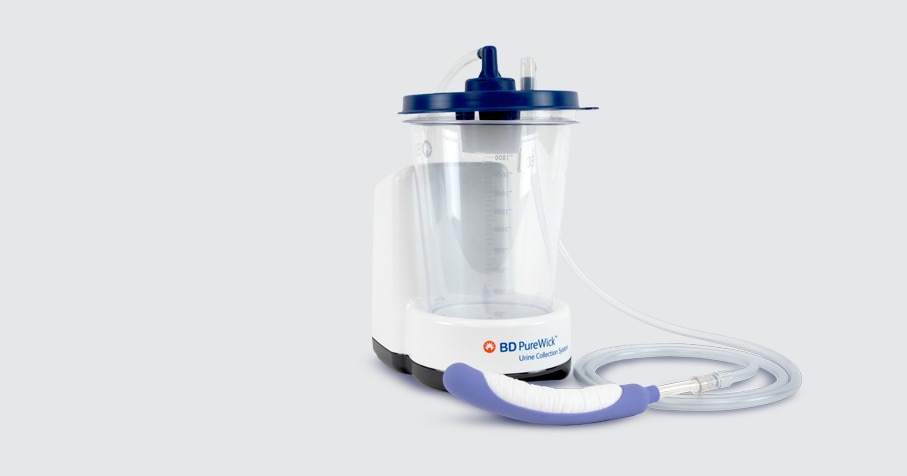
The PureWick™ Urine Collection System is designed to work with PureWick™ External Catheters to wick voided urine away from the body, into a designated collection canister. Patients can benefit from the PureWick™ Urine Collection System both in a health care setting and at home.
PureWick™ Urine Collection System
From hospital to home, the PureWick™ System is an innovative option to managing urinary incontinence.

- Overview
- Products & Accessories
- EIFU & Resources
The PureWick™ System can support your patients across the continuum of care in both home environments and professional care facilities.
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.
We support the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
The BD Learning Academy offers a centralized repository of BD product training, education, and the ability to connect courses to your LMS.
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
Indications for Use: The PureWick™ Urine Collection System is to be used with PureWick™ External Catheters which are intended for non-invasive urine output management.
Contraindications for Use: Do not use the PureWick™ Urine Collection System with PureWick™ External Catheters on individuals with urinary retention.
Safety and Warnings: Always unplug PureWick™ Urine Collection System before cleaning or when not in use.
DO NOT IMMERSE THE PUREWICK™ URINE COLLECTION SYSTEM IN WATER.
As with most electrical devices, electrical parts in this system are electrically live even when the power is off. To reduce the risk of electric shock, if the PureWick™ Urine Collection System falls into water, unplug immediately. DO NOT REACH
INTO THE WATER TO RETRIEVE IT.
WARNING: Contains small parts that may cause choking. Keep out of reach of children. Keep cords and tubing out of the reach of children to avoid the risk of strangulation. Discontinue use if an allergic reaction occurs. Not recommended for users who are experiencing skin irritation or skin breakdown in device contact areas.
WARNING: This device should not be used in oxygen rich environments or in conjunction with flammable anesthetics. If the PureWick™ Urine Collection System is dropped or tipped over spilling urine, unplug the unit and carefully inspect for loose or damaged parts before resuming use. Contact Customer Support at 1-888-201-1586 if any damage is observed. The pump tubing connecting the PureWick™ Urine Collection System to the collection canister may experience light condensation inside the tube. This is not unusual and does not affect function. However, if urine or water has streamed into the pump, discontinue use and contact Customer Support at 1-888-201-1586.
Although the canister can hold up to 2000cc (mL), the urine should be emptied regularly from the collection canister before volume reaches 1800cc (mL). Failure to empty canister before urine overflow may cause damage to the PureWick™ Urine Collection System and is not covered under the warranty. It is important that the port connections be connected correctly for proper operation of the PureWick™ Urine Collection System. Use of this equipment next to or stacked with other equipment should be avoided because it could result in improper operation. If such use is necessary, this equipment and the other equipment should be observed to verify that they are operating normally. Portable radio frequency (RF) communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 12 inches (30cm) to any part of the PureWick™ Urine Collection System, including cables specified by the manufacturer. Otherwise, degradation of the performance of this equipment could result. Use only PureWick™ Urine Collection System accessories with this device. Incompatible parts or accessories can result in degraded performance and will void the warranty. Do not use accessories past expiration date indicated on package labeling. Use only the PureWick™ Urine Collection System A/C power cord with the device. Use of an alternate consumer style A/C power adapter or an extension cord may cause damage to device and electrical shock or injury and will void the warranty. Do not place PureWick™ Urine Collection System or its cord across walkways creating a tripping hazard.
PureWick™ Female External Catheter indications: The PureWick™ Female External Catheter is intended for non-invasive urine output management iin female patients.
Contraindications: The PureWick™ Female External Catheter should not be used on patients with urinary retention.
Warnings: To avoid potential skin injury, never push or pull the PureWick™ Female External Catheter inot the vagina, anal canal or other body cavities. Do not use the PureWick™ Female External Catheter with a bedpan or any material that does not allow for sufficient airflow. Discontinue use of an allergic reaction occurs. After use, the product may be a potential biohazard. Dispose of in accordance with applicable local, state and federal laws an regulations.
For the latest information, always check the "Instructions for Use" that comes packaged with the product. If you are a consumer seeking more information. please consult your healthcare provider.
PureWick™ Flex Female External Catheter indications: The PureWick™ Flex Female External Catheter is intended for non-invasive urine output management in adult users with female anatomy, for conditions such as urinary incontinence.
Contraindications: The PureWick™ Flex Female External Catheter should not be used on users with urinary retention.
Warnings: The PureWick™ Flex Female External Catheter is designed for single-use only. Trying to wash, sanitize or reuse it may lead to risk of infection or illness. This may damage the product and materials. The product may not work and cause injury or illness. To avoid potential skin injury, never push or rub the product against the skin during placement or removal. Do not block airflow to the product (e.g., blocking the vent hole, bedpan, barrier creams). Do not insert the product into vagina, anus, or other body orifice. Stop use if an allergic reaction occurs. Do not use on users with frequent episodes of stool incontinence without a fecal management system in place. Do not use on users with skin irritation or breakdown at the site. Use with caution on users who had recent surgery of the external female anatomy. Do not use on users with moderate/heavy menstruation who cannot use a tampon. After use, this product may be a potential biohazard. Dispose of in accordance with applicable local, state, and federal laws and regulations.
PureWick™ Male External Cathter indications: The PureWick™ Male External Catheter is intended for non-invasive urine output management in male patients.
Contraindication: Patients with urinary retention
Precaution: Not recommended for patients who are: Agitated, combative, or uncooperative and might remove the device. Having frequent episodes of bowel incontinence without a fecal management system in place. Experiencing skin irritation or breakdown at the site. Do not use barrier cream on the penis or pubic skin around the base of the penis when using the device. Creams may impede suction or weaken adhesive. Proceed with caution in patients who have undergone recent surgery of the external urogenital tract, penis, or pubic area. Always assess skin for compromise and perform perineal care prior to placement of a new device.
Warning: Do not use on irritated or compromised skin. Do not cover fresh surgical wounds with the device. Do not use the device with any material or in any position that does not allow for sufficient airflow. To avoid potential skin injury, never pull the device directly away from the patient. Always peel in the direction from head to foot. Discontinue use if an allergic reaction occurs. After use, this product may be a potential biohazard. Dispose of in accordance with applicable local, state, and federal laws and regulations.
PureWick is a trademark of Becton, Dickinson and Company or its affiliates.
© 2025 BD. All rights reserved.

