Safety and Risk Information
Indication for Use
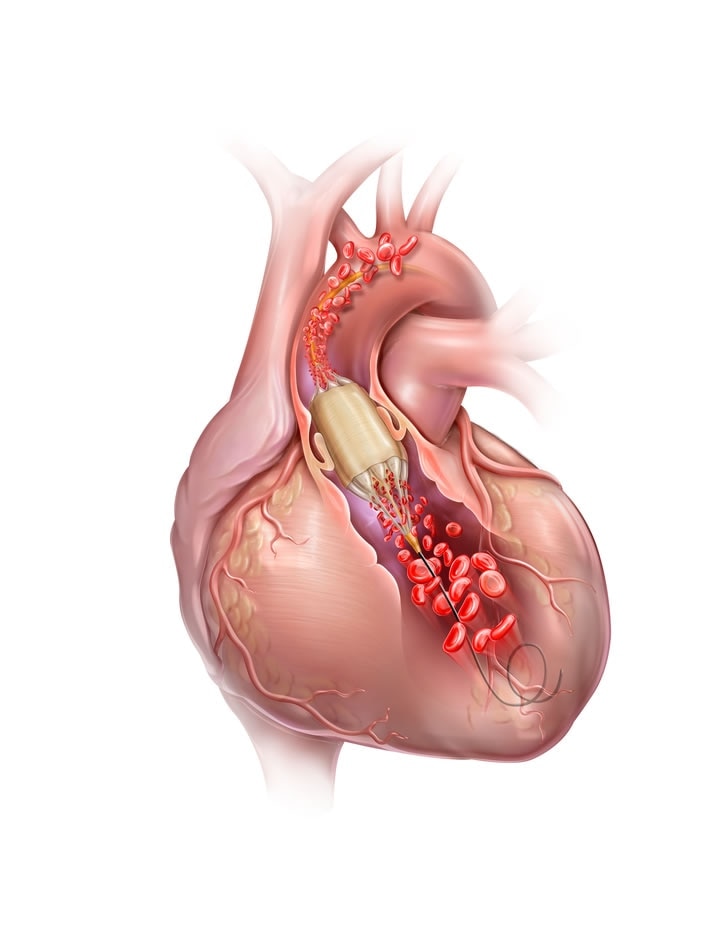
The True™ Flow Valvuloplasty Perfusion Catheter is indicated for balloon aortic valvuloplasty.
Contraindications
None know
Warnings
1. Do not use in patients with annular dimensions <18mm.
2. Contents supplied STERILE using ethylene oxide (EO). Non-pyrogenic. Do not use if sterile barrier is opened or damaged. Single patient use only. Do not reuse, reprocess or re-sterilize.
3. This device has been designed for single use only. Reusing this medical device bears the risk of cross-patient contamination as medical devices – particularly those with long and small lumina, joints, and/or crevices between components – are difficult or impossible to clean once body fluids or tissues with potential pyrogenic or microbial contamination have had contact with the medical device for an indeterminable amount of time. The residue of biological material can promote the contamination of the device with pyrogens or microorganisms which may lead to infectious complications.
4. Do not resterilize. After resterilization, the sterility of the product is not guaranteed because of an indeterminable degree of potential pyrogenic or microbial contamination which may lead to infectious complications. Cleaning, reprocessing and/or resterilization of the present medical device increases the probability that the device will malfunction due to potential adverse effects on components that are influenced by thermal and/or mechanical changes.
5. Catheter balloon inflation diameter must be carefully considered in selecting a particular size for any patient. It is critical to perform a clinical diagnostic determination of valve anatomical dimensions prior to use; imaging modalities such as transthoracic echocardiogram (TTE), computerized tomography (CT), angiography, and/or transesophageal echocardiogram (TEE) should be considered. The inflated balloon diameter should not be significantly greater than valvular diameter.
6. When the catheter is exposed to the vascular system, it should be manipulated while under high-quality fluoroscopic observation. Do not advance or retract the catheter unless the balloon is fully deflated. If resistance is met during manipulation, determine the cause of the resistance before proceeding. Applying excessive force to the catheter can result in tip breakage or balloon separation, or cause injury to the patient (such as vessel perforation).
7. If flow through catheter becomes restricted, do not attempt to clear catheter lumen by infusion. Doing so may cause catheter to rupture, resulting in vessel trauma. Remove and replace catheter.
8. Do not exceed the RBP recommended for this device. Balloon rupture may occur if the RBP rating is exceeded. To prevent over-pressurization, use of a pressure monitoring device is recommended.
9. After use, this product may be a potential biohazard. Handle and dispose of in accordance with acceptable medical practices and applicable local, state, and federal laws and regulations. 10. If using device to support Transcatheter Aortic Valve Implantation (TAVI), consult TAVI system’s Instructions for Use for any additional procedural instructions related to selection and use of valvuloplasty balloon. 11. To reduce thrombosis, this device should not be used without appropriate anticoagulation. It is recommended to maintain an ACT of ≥200 seconds during use of this device.
Precautions
1. Carefully inspect the catheter prior to use to verify that catheter has not been damaged during shipment and that its size, shape and condition are suitable for the procedure for which it is to be used. Do not use if product damage is evident.
2. The minimal acceptable French size is printed on the package label. Do not attempt to pass the catheter through a smaller size introducer sheath than indicated on the label.
3. Use the recommended balloon inflation medium of 1/3 to 2/3 contrast to saline ratio. Never use air or other gaseous medium to inflate the balloon.
4. If resistance is felt during post procedure withdrawal of the catheter through the introducer sheath, determine if contrast is trapped in the balloon with fluoroscopy. If contrast is present, push the balloon out of the sheath and then completely evacuate the contrast before proceeding to withdraw the balloon.
5. If resistance is still felt during post procedure withdrawal of the catheter, it is recommended to remove the balloon catheter and guidewire/introducer sheath as a single unit.
6. In the very unlikely event of balloon burst or rupture, balloon could be more difficult to remove through the sheath and could require introducer sheath removal.
7. Do not torque, excessively bend catheter or continue to use if the shaft has been bent or kinked.
8. Do not re-insert the catheter into the body once it has been removed from the sheath, as withdrawing balloon through introducer sheath may damage balloon.
9. Do not remove guidewire from catheter during procedure.
10. Dilation procedures should be conducted under high-quality fluoroscopic guidance.
11. Careful attention must be paid to the maintenance of tight catheter connections. Aspirate before proceeding to avoid air introduction into the system.
12. If inflating balloon in patient to facilitate re-folding, ensure balloon is positioned so that it can be inflated safely.
Potential Complications
The complications which may result from a percutaneous transluminal valvuloplasty procedure include:
Additional intervention • Allergic reaction to drugs or contrast medium • Aneurysm or pseudoaneurysm • Arrhythmias • Cardiovascular injury • Conduction system injury • Death • Embolization • Hematoma • Hemorrhage, including bleeding at the puncture site • Hypotension/hypertension • Inflammation • Occlusion • Pain or tenderness • Pneumothorax or hemothorax • Sepsis/infection • Shock • Short term hemodynamic deterioration • Stroke • Thrombosis • Valvular tearing or trauma • Vessel dissection, perforation, rupture, or spasm
Please consult the package insert for more detailed safety information and instructions for use.
BD, the BD logo, and True are trademarks of Becton, Dickinson and Company or its affiliates. © 2023 BD. All rights reserved. | 1 800 321 4254 | www.bd.com | 1625 W.3rd Street Tempe, AZ 85281
BD-111052