- BD BACTEC™ Plus Aerobic and Lytic Anaerobic media accelerate time to detection, improve recovery of aerobic and anaerobic pathogens - enabling faster, more targeted antimicrobial therapy.8,10,11,14
BD BACTEC™ Blood Culture System
Every second counts—BD BACTEC™ ensures critical answers arrive when they’re needed most, helping save lives.


- Overview
- Products & Accessories
- EIFU & Resources
Every minute—and every decision—matters
When bloodstream infections and sepsis strike, every second counts. Properly diagnosing a bloodstream infection is critical, and delays in accurate results can cost lives. Every false positive, every missed pathogen, every delay in targeted antimicrobial therapy can increase patient mortality by up to 50%.¹ That’s why the BD BACTEC™ Blood Culture Solution is built to protect what matters most—patients. By simplifying blood culture collection, accelerating detection, and ensuring reliable results, BD empowers clinicians to act fast with confidence. From driving efficiency and improving sample integrity during collection to unique media that recover even the toughest pathogens, modular instruments, and dynamic informatics, BD delivers a complete diagnostic pathway focused on what matters most—better patient outcomes.
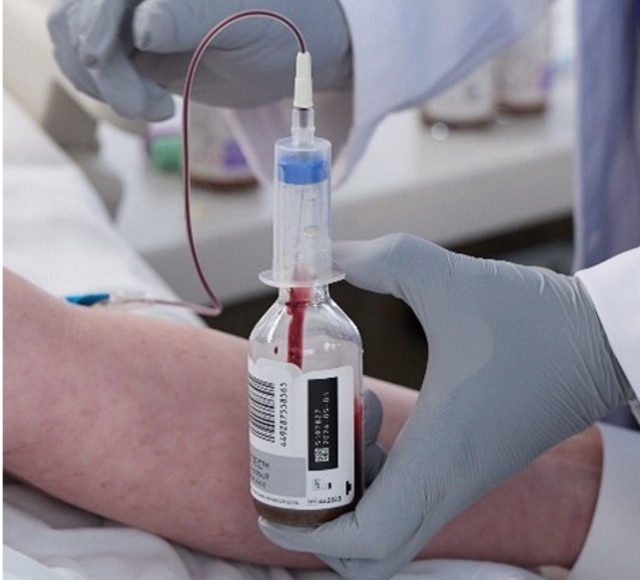
Efficiency that impacts lives: Simplify collection, accelerate detection
Streamline your blood culture collection process to avoid delays in patient care, lab rework, and costly redraws. 2-5 Because an efficient bloodstream infection diagnostic pathway begins with high-quality blood culture samples. 6,7
BD BACTEC™ fits seamlessly into your existing phlebotomy procedure:
- Removes need for additional adapters/holders
- Minimizes contamination touchpoints
- Reduces inventory requirements and waste
- Potentially reduces risk of needlestick injuries
The BD BACTEC™ Blood Culture System simplifies the phlebotomy procedure by reducing steps and minimizing contamination touchpoints – driving efficiency and improving sample integrity.

Coverage against the toughest threats
BD offers a complete line of blood culture media designed to recover even the most challenging organisms, ensuring detection of aerobes, anaerobes, facultative anaerobes, yeast, fungi and mycobacteria, all specifically developed to help improve time to detection and organism recovery, supporting timely life-saving decisions.8- 11,14

Simple. Reliable. Modular.
In today’s microbiology laboratories, these qualities aren’t optional—they’re critical. BD BACTEC™ FX instruments are fit for your purpose, delivering simplicity in operation and ease of adoption, seamlessly integrating into your daily workflow. Designed with growth in mind, these systems expand as your laboratory’s needs evolve.
The modular architecture of BD BACTEC™ FX empowers you to centralize testing in a core lab or decentralize incubation for faster time to detection—without compromising consistency or quality. Across the continuum of care, from small community hospitals to large reference labs, BD BACTEC™ FX ensures the same high-performance standards, enabling reliable blood culture testing wherever it’s needed.
BD BACTEC™ FX: Built for today. Ready for tomorrow.
Media that match the microbe, unique formulations
to recover what others might miss
- Because almost 50% of patients are already receiving antibiotics before the first blood culture is drawn, selecting a blood culture medium with proven antimicrobial neutralization is critical to ensure accurate pathogen recovery and timely diagnosis12
- BD BACTEC™ Plus Aerobic Medium contains two resins, light and dark, with varying absorption capabilities to cover more antibiotic classes to enhance recovery of aerobic bacteria and yeast
- Recently, BD modified the formulation to further enhance recovery and time to detection for both bacteria and yeast13
- Enhanced recovery of anaerobes through targeted cell lysis
- Contains saponin to lyse red and white blood cells –releasing intracellular organisms and enriching the medium to support robust microbial growth
- Proven performance in the detection of rare, but deadly anaerobic organisms which make up 1-17% of all positive blood cultures but are associated with 15-30% of the mortality rate8,10.14
- Uniquely formulated to detect critical pediatric pathogens
- Superior recovery and time to detection of Kingella kingae15
- Accommodates small-volume samples (0.5-5.0 mL)
- Designed for recovery of yeast and fungi from sterile body fluids and blood specimens
- Enables recovery of mycobacterium from blood specimens
BD Synapsys™ Informatics: Turning data into decisions

Unlock the power of dynamic intelligence with BD Synapsys™ Informatics. Transform complex clinical microbiology data into timely, actionable insights—empowering your lab to optimize operations, drive quality improvement initiatives, and deliver accurate results to clinicians faster.
With BD Synapsys™, you gain more than data—you gain clarity, efficiency, and confidence across your entire bloodstream infection diagnostic pathway from specimen collection to actionable result.
BD BACTEC™—For the fight back.
The clock is ticking—Act now!

From the moment a blood culture is drawn, every second matters. Behind every test is a patient hoping for healing, a family waiting for answers, a life depending on timely care. They’re counting on you—and we’re here to help.
Join the fight against bloodstream infections and sepsis with the BD BACTEC™ Blood Culture Solution.
Contact us today to learn how BD BACTEC™ can help you deliver critical answers faster. Download the Brochure for details on building a more efficient diagnostic pathway.
Related Products
An efficient bloodstream infection diagnostic pathway begins with high-quality blood culture samples8,9
From the detection of a positive blood culture, BD offers a full suite of identification and antimicrobial susceptibility testing solutions to deliver actionable results
Meaningful and actionable insights accessible anytime, anywhere with BD Informatics
- Kadri SS, Lai YL, Warner S, Strich JR, Babiker A, Ricotta EE, Demirkale CY, Dekker JP, Palmore TN, Rhee C, Klompas M, Hooper DC, Powers JH 3rd, Srinivasan A, Danner RL, Adjemian J; forming the National Institutes of Health Antimicrobial Resistance Outcomes Research Initiative (NIH-ARORI). Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: a retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals. Lancet Infect Dis. 2021 Feb;21(2):241-251
- CLSI Guideline M47. Clinical and Laboratory Standards Institute; 2022. ,
- CDC. Preventing Adult Blood Culture Contamination: A Quality Tool for Clinical Laboratory Professionals.
- Doern GV et al. Clin Microbiol Rev. 2020;33(1):e00009–19.
- Halverson S et al. J Clin Microbiol. 2013;51(6):1721–6..
- Lamy B et al. Front Microbiol. 2016;7:697.
- CDC. Preventing Adult Blood Culture Contamination: A Quality Tool for Clinical Laboratory Professionals. Available at: https://www.cdc.gov/labquality/docs/BCC-Prevention_A-Quality-Tool_CDC.pdf. Accessed October 2023.
- Li G et al. Front Cell Infect Microbiol. 2019;9:285.
- Zadroga R et al. Clin Infect Dis. 2013;56(6):790–7.
- Almuhayawi M et al. PLoS ONE. 2015;10(11):e0142398.
- Rocchetti A et al. J Microbiol Methods. 2016;130:129–32.
- Sayon Dutta, Dustin S McEvoy, David M Rubins, Anand S Dighe, Michael R Filbin, Chanu Rhee, Clinical decision support improves blood culture collection before intravenous antibiotic administration in the emergency department, Journal of the American Medical Informatics Association, Volume 29, Issue 10, October 2022, Pages 1705–1714, https://doi.org/10.1093/jamia/ocac115
- U.S. Food and Drug Administration. (2023). Premarket Notification (510(k)) for BD BACTEC™ Plus Aerobic/F Culture Vials (K222591). Silver Spring, MD: U.S. Department of Health and Human Services. Retrieved from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm
- Rohner P et al. J Clin Microbiol. 1997;35(10):2634–8.
- Yarbrough ML, Wallace MA, Burnham CD. Comparison of Microorganism Detection and Time to Positivity in Pediatric and Standard Media from Three Major Commercial Continuously Monitored Blood Culture Systems. J Clin Microbiol. 2021 Jun 18;59(7):e0042921. doi: 10.1128/JCM.00429-21. Epub 2021 Jun 18. PMID: 33910963; PMCID: PMC8218758.
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.






