Sort by:
true
SenoMark™ UltraCor™ Breast Tissue Marker
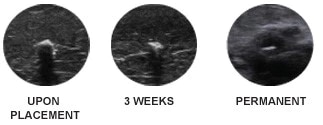
This rigid marker is designed to assist in accurate placement and to provide remarkable ultrasound visibility for 3 weeks with permanent ultrasound enhancement.

- Overview
- Products & Accessories
- EIFU & Resources
IT STANDS OUT
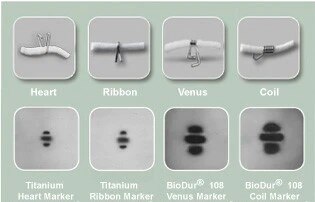
The SenoMark™ UltraCor™ Breast Tissue Markers are designed to provide remarkable ultrasound visibility for three weeks with permanent ultrasound enhancement. Four unique shapes clearly mark multiple sites within the same breast, each with a different level of MR visualization.
Ultrasound: It's what you are looking for
Polyglycolic acid (PGA) microfiber absorbent pads provide 3 weeks of ultrasound visibility. Non-absorbable polyvinyl alcohol (PVA) polymer, interwoven with a wireform, provides permanent ultrasound enhancement.
MRI: Four clear choices
Four unique shapes, made of two different metals, provide a wide choice of MR visualization options.
Designed for accurate placement and minimal migration
- Resorbable Pads: PGA microfiber absorbent pads are designed for accurate placement and minimal migration until they are resorbed in approximately 12 weeks.
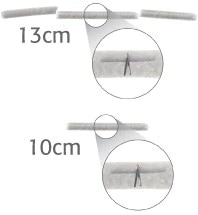
- 13 cm Rigid Applicator: Uses 3 pads, with the proximal and distal pads designed to keep the center pad, containing a wireform interwoven with PVA.
- 10 cm Rigid Applicator: Uses 1 PGA pad containing a wireform interwoven with PVA.
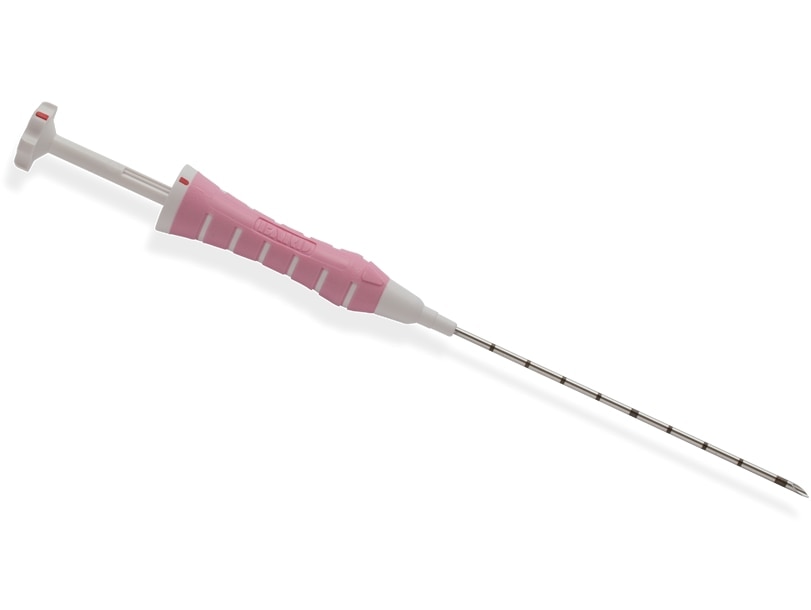
Versatile applicator
The SenoMark™ UltraCor™ applicator is packaged with adapters that may be used with:
- EnCor Enspire™ Breast Biopsy System
- EnCor™ MRI Introducer Probe
- Eviva® Breast Biopsy System

Marker Shapes that Celebrate Her
With each purchase of the Heart, Venus or Ring breast tissue marker shapes, BD will contribute $1 to the American Cancer Society™ in honor of breast cancer patients.**
* Applies to specially marked BD breast tissue marker products purchased from BD. The American Cancer Society™ does not endorse any service or product. Visit bd.com/icoh for details. Valid through September 30.
Please consult product labels and inserts for indications, contraindications, hazards, warnings, precautions and directions for use.
BD-110459v2
true
Literature
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.
Learn more
Events
We support the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Learn more
Training
The BD Learning Academy offers a centralized repository of BD product training, education, and the ability to connect courses to your LMS.
Learn More
Case Studies
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
Learn more
true
true