-
Highlander™ 014 PTA Balloon Dilatation Catheter
4 mm x 80 mm Balloon, 150 cm long catheter
SKU/REF h415048
-
Highlander™ 014 PTA Balloon Dilatation Catheter
4 mm x 60 mm Balloon, 150 cm long catheter
SKU/REF h415046
-
Highlander™ 014 PTA Balloon Dilatation Catheter
4 mm x 40 mm Balloon, 150 cm long catheter
SKU/REF h415044
-
Highlander™ 014 PTA Balloon Dilatation Catheter
4 mm x 20 mm Balloon, 150 cm long catheter
SKU/REF h415042
-
Highlander™ 014 PTA Balloon Dilatation Catheter
4 mm x 150 mm Balloon, 150 cm long catheter
SKU/REF h4150415
-
Highlander™ 014 PTA Balloon Dilatation Catheter
4 mm x 120 mm Balloon, 150 cm long catheter
SKU/REF h4150412
-
Highlander™ 014 PTA Balloon Dilatation Catheter
4 mm x 100 mm Balloon, 150 cm long catheter
SKU/REF h4150410
-
Highlander™ 014 PTA Balloon Dilatation Catheter
3.5 mm x 80 mm Balloon, 150 cm long catheter
SKU/REF h41503h8
-
Highlander™ 014 PTA Balloon Dilatation Catheter
3.5 mm x 60 mm Balloon, 150 cm long catheter
SKU/REF h41503h6
-
Highlander™ 014 PTA Balloon Dilatation Catheter
3.5 mm x 40 mm Balloon, 150 cm long catheter
SKU/REF h41503h4
-
Highlander™ 014 PTA Balloon Dilatation Catheter
3.5 mm x 20 mm Balloon, 150 cm long catheter
SKU/REF h41503h2
-
Highlander™ 014 PTA Balloon Dilatation Catheter
3.5 mm x 150 mm Balloon, 150 cm long catheter
SKU/REF h41503h15
-
Highlander™ 014 PTA Balloon Dilatation Catheter
3.5 mm x 120 mm Balloon, 150 cm long catheter
SKU/REF h41503h12
-
Highlander™ 014 PTA Balloon Dilatation Catheter
3.5 mm x 100 mm Balloon, 150 cm long catheter
SKU/REF h41503h10
-
Highlander™ 014 PTA Balloon Dilatation Catheter
3 mm x 80 mm Balloon, 150 cm long catheter
SKU/REF h415038
-
Highlander™ 014 PTA Balloon Dilatation Catheter
3 mm x 60 mm Balloon, 150 cm long catheter
SKU/REF h415036
-
Highlander™ 014 PTA Balloon Dilatation Catheter
3 mm x 40 mm Balloon, 150 cm long catheter
SKU/REF h415034
-
Highlander™ 014 PTA Balloon Dilatation Catheter
3 mm x 20 mm Balloon, 150 cm long catheter
SKU/REF h415032
-
Highlander™ 014 PTA Balloon Dilatation Catheter
3 mm x 150 mm Balloon, 150 cm long catheter
SKU/REF h4150315
-
Highlander™ 014 PTA Balloon Dilatation Catheter
3 mm x 120 mm Balloon, 150 cm long catheter
SKU/REF h4150312
-
Highlander™ 014 PTA Balloon Dilatation Catheter
3 mm x 100 mm Balloon, 150 cm long catheter
SKU/REF h4150310
-
Highlander™ 014 PTA Balloon Dilatation Catheter
2.5 mm x 80 mm Balloon, 150 cm long catheter
SKU/REF h41502h8
-
Highlander™ 014 PTA Balloon Dilatation Catheter
2.5 mm x 60 mm Balloon, 150 cm long catheter
SKU/REF h41502h6
-
Highlander™ 014 PTA Balloon Dilatation Catheter
2.5 mm x 40 mm Balloon, 150 cm long catheter
SKU/REF h41502h4
-
Highlander™ 014 PTA Balloon Dilatation Catheter
2.5 mm x 20 mm Balloon, 150 cm long catheter
SKU/REF h41502h2
-
Highlander™ 014 PTA Balloon Dilatation Catheter
2.5 mm x 150 mm Balloon, 150 cm long catheter
SKU/REF h41502h15
-
Highlander™ 014 PTA Balloon Dilatation Catheter
2.5 mm x 120 mm Balloon, 150 cm long catheter
SKU/REF h41502h12
-
Highlander™ 014 PTA Balloon Dilatation Catheter
2.5 mm x 100 mm Balloon, 150 cm long catheter
SKU/REF h41502h10
-
Highlander™ 014 PTA Balloon Dilatation Catheter
2 mm x 80 mm Balloon, 150 cm long catheter
SKU/REF h415028
-
Highlander™ 014 PTA Balloon Dilatation Catheter
2 mm x 60 mm Balloon, 150 cm long catheter
SKU/REF h415026
-
Highlander™ 014 PTA Balloon Dilatation Catheter
2 mm x 40 mm, Balloon, 150 cm long catheter
SKU/REF h415024
-
Highlander™ 014 PTA Balloon Dilatation Catheter
2 mm x 20 mm Balloon, 150 cm long catheter
SKU/REF h415022
-
Highlander™ 014 PTA Balloon Dilatation Catheter
2 mm x 150 mm Balloon, 150 cm long catheter
SKU/REF h4150215
-
Highlander™ 014 PTA Balloon Dilatation Catheter
2 mm x 120 mm Balloon, 150 cm long catheter
SKU/REF h4150212
-
Highlander™ 014 PTA Balloon Dilatation Catheter
2 mm x 100 mm Balloon, 150 cm long catheter
SKU/REF h4150210
Highlander™ 014 PTA Balloon Dilatation Catheter
The Fiberplasty Difference in Small-Vessel PTA

- Overview
- Products & Accessories
- EIFU & Resources
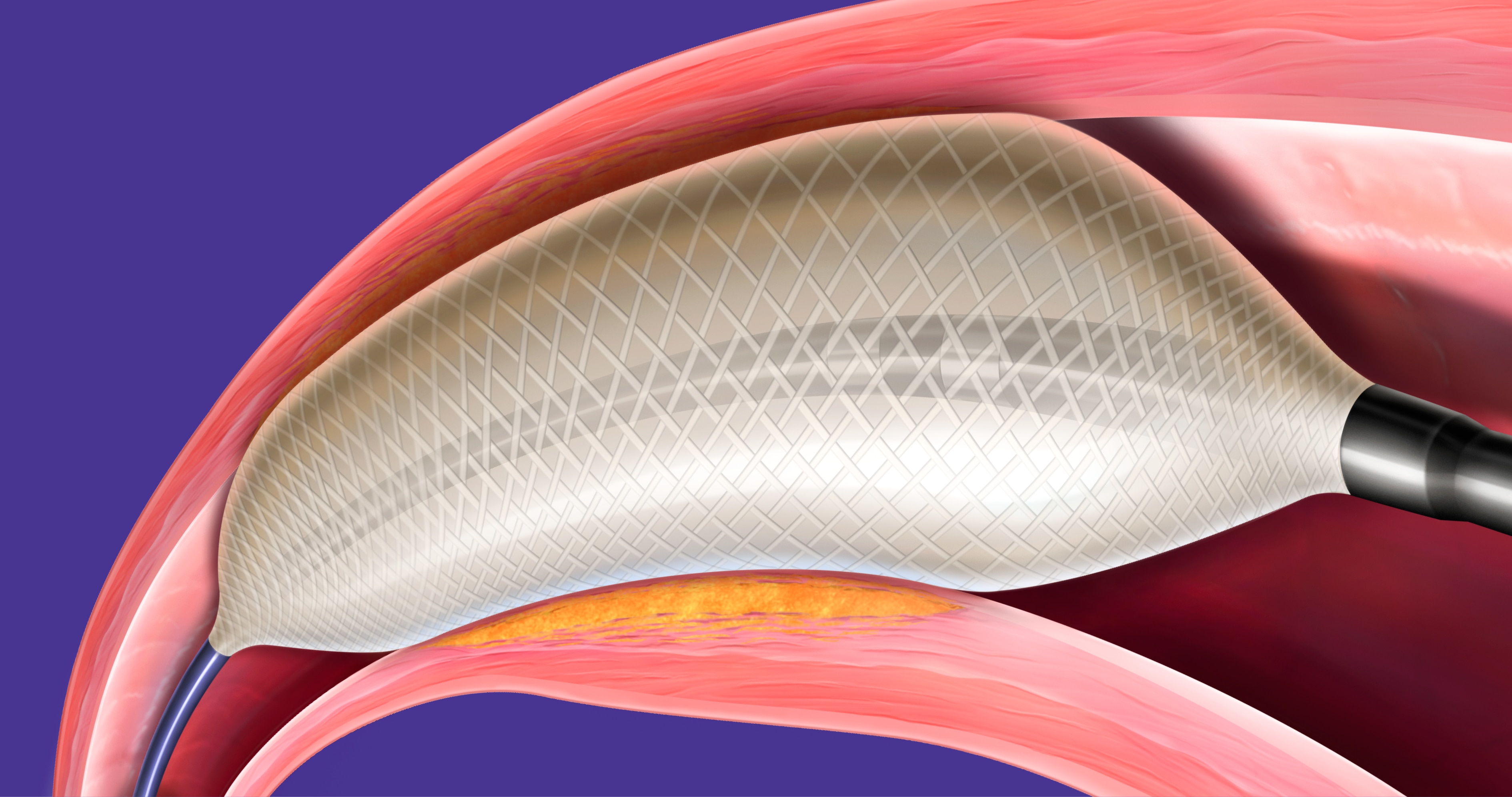
The Highlander™ 014 PTA Balloon Dilatation Catheter is a fiber-based, non-compliant balloon that is designed to deliver highly precise diameters and lengths relative to labeled sizing, even at high pressures.
- Highly Precise Sizing
- In bench testing, the Highlander™ 014 PTA Balloon Dilatation Catheter provided more precise sizing relative to labeled diameter, on average, at both Nominal Pressure and Rated Burst Pressure (RBP) compared to select leading competitors. 1
- Highly Controlled Compliance
- In bench testing, the Highlander™ 014 PTA Balloon Dilatation Catheter had significantly less outer diameter and longitudinal growth, on average, between Nominal Pressure and RBP compared to select leading competitors. 2
- Unmatched Strength
- The Highlander™ 014 PTA Balloon Dilatation Catheter has the largest working pressure range and highest RBP of any 0.014 or 0.018 PTA balloon. 3
- First and only fiber-based non-compliant balloon available on an 0.014 guidewire platform. 3
- Low Profile
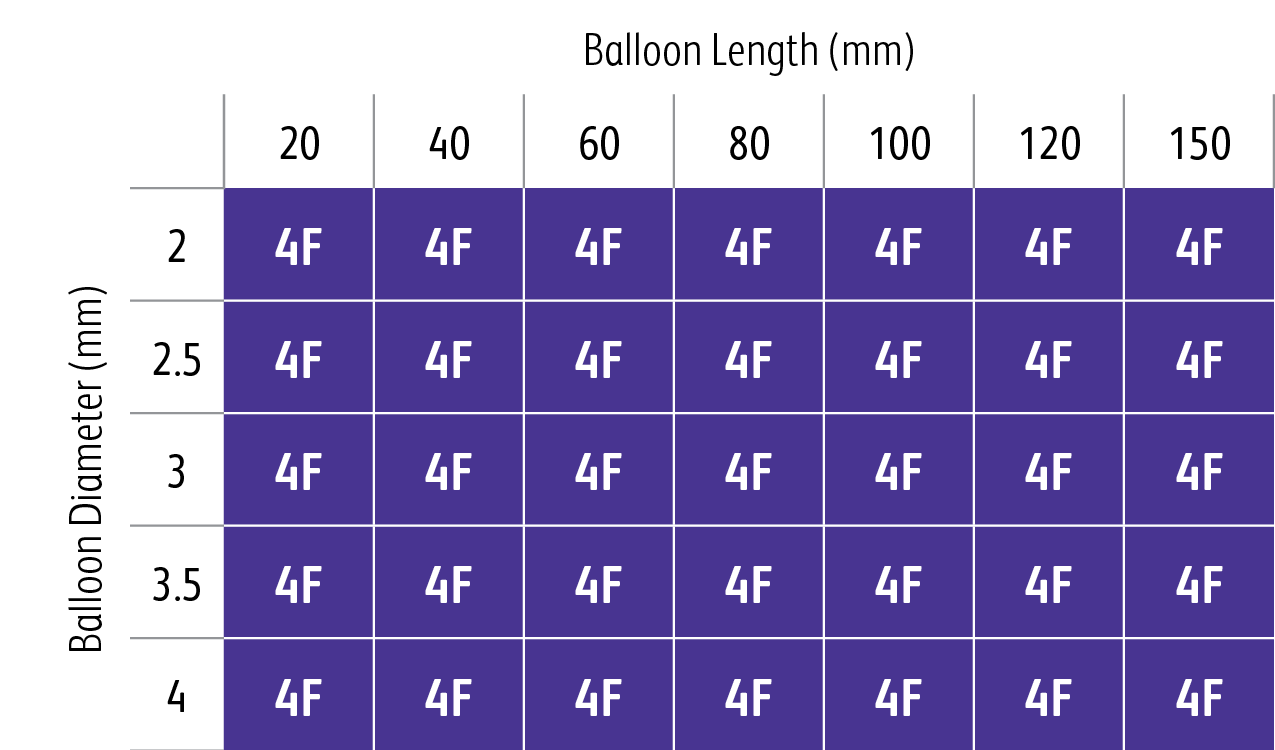
- The Highlander™ 014 PTA Balloon has 4F sheath compatibility across all sizes.
Features designed to help facilitate effective PAD treatment in small vessels:
- Low tip entry profile designed to facilitate crossing tight lesions
- Lubricious coating designed to enhance trackability
- Fiber-based balloon technology is designed to help minimize the "dog-bone" effect
The Highlander™ 014 PTA Balloon Dilatation Catheter consists of a 150 cm-length, over-the-wire catheter compatible with 0.014” guidewires. It offers a variety of balloon sizes targeted for infrapopliteal angioplasty.
The GeoAlign™ Marking System is a non-radiopaque ruler on the catheter shaft measured from the distal tip. During repeat catheter placement, the GeoAlign™ Marking System is designed to:
- Help increase procedure efficiency and decrease radiation exposure by minimizing fluoroscopy time
- Facilitate repeat catheter alignment at the lesion
- Simplify length measurement between two intravascular points
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.
We support the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
The BD Learning Academy offers a centralized repository of BD product training, education, and the ability to connect courses to your LMS.
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
1 Accuracy measurements based on variance in balloon outer diameter between Nominal Pressure (NP) and Rated Burst Pressure (RBP) compared to labeled diameter for 2 mm balloons. Balloon outer diameters are measured at center of the balloon at NP and at RBP. Mean results reported in mm, as follows: Highlander™ 014 Balloon (NP: 1.97, RBP: 2.00); Armada™ 14 (NP: 1.88, RBP: 2.01); Coyote™ (NP: 1.82, RBP: 2.02); and Pacific Plus™ (NP: 2.04, RBP: 2.32). All balloons tested were 2x150 mm, besides Armada™ 14 which was 2x200 mm. N=5 for all balloons tested. Data on file. BD, Tempe, AZ. Bench data may not be indicative of actual clinical performance. Different test methods may yield different results.
2 Outer diameter measurements taken from center of balloon at NP and RBP. Mean results reported for percentage outer diameter growth, as follows: Highlander™ 014 Balloon (2.37%); Jade™ 014 (4.73%); Armada™ 14 (7.49%); Nanocross™ (9.02%); and Coyote™ (14.93%). Length measurements taken from point of cone taper at proximal and distal ends of the balloon at NP and RBP. Mean results reported for percentage longitudinal growth, as follows: Highlander™ 014 Balloon (0.67%); Jade™ 014 (2.18%); Armada™ 14 (2.30%); Nanocross™ (2.58%); and Coyote™ (2.84%). All balloons tested were 4x150 mm, besides Armada™ 14 which was 4x200 mm. N=5 for all balloons tested. Bench test data on file. BD, Tempe, AZ. Bench test results may not necessarily be indicative of clinical performance. Different test methods may yield different results.
3 As of January 2023. US market only. EVT Device Guide, PTA Balloons. Competitive balloon material information obtained from product web pages and/or 510(k) summaries.
Please consult product labels and instruction for use for indications, contraindications, hazards, warnings and precautions.
BD-81022
