†Compared to vascular access

- Overview
- Products & Accessories
The only integrated peripheral IV catheter system available in the
U.S. with indication to deliver the benefits of subcutaneous infusion
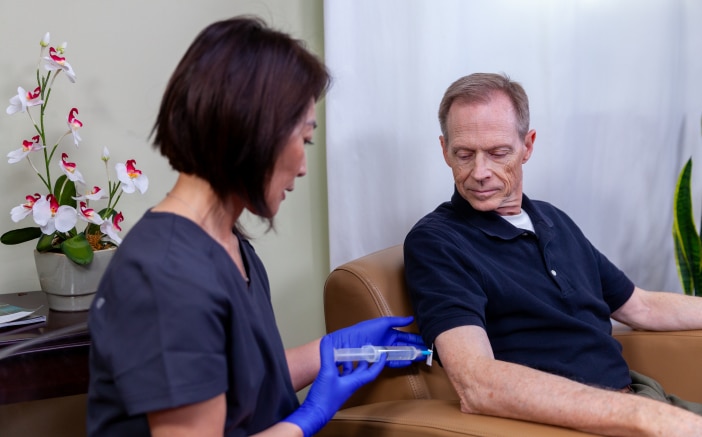
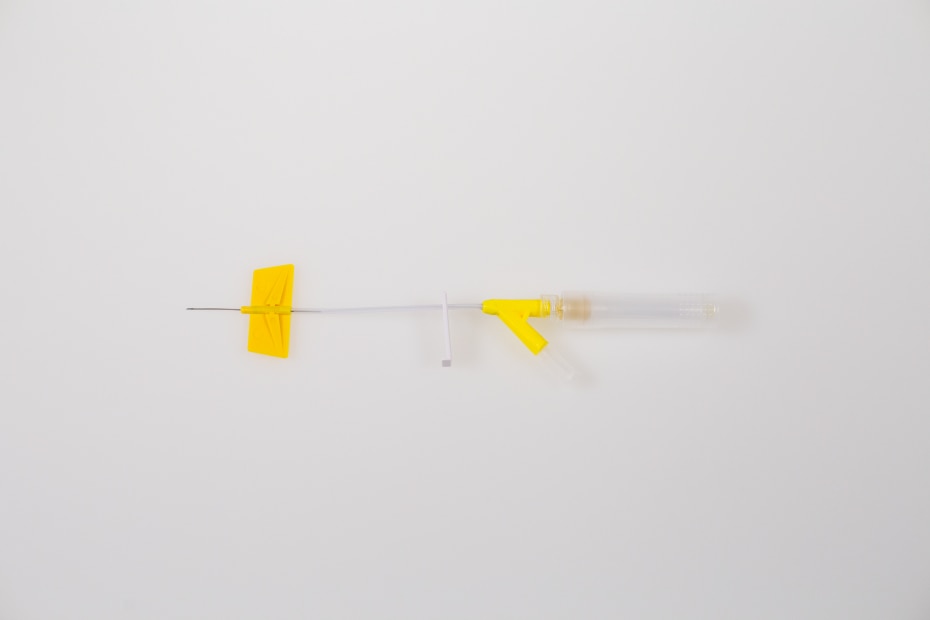
BD® Saf-T-Intima™ Subcutaneous Catheter System is an over-the-
needle, integrated IV catheter. The closed system is designed to keep
blood contained within the device throughout the insertion process.
The needle is passively protected when it is removed.
The Saf-T-Intima™ System is designed for subcutaneous infusion.
When clinically indicated, subcutaneous infusion can address the
challenges of vascular access as an effective alternative for patients,
especially those who may have difficult venous access (DIVA).1,2
Subcutaneous access can play a part in the support of a patient’s vessel
health and preservation.1,3
- Low-profile, flexible wings designed to help with patient comfort
- Extension tube designed to help minimize catheter movement during
administration of therapy* - Proprietary BD® Vialon™ Catheter Material
*Compared to administration with a needle and syringe
Subcutaneous infusion has been shown to provide:†
Increased placement success1,4
Improved patient comfort and satisfaction2,5
Increased infusion center efficiencies2,5
Lower risk of complications1,2
Reduced clinician placement time1,2,5
Lower cost to deliver therapy1
Designed to meet clinicians' needs across various care settings
- Broadhurst D, Cooke M, Sriram D, et al. Subcutaneous hydration and medications infusions (effectiveness, safety, acceptability): a systematic review of systematic reviews. PLoS ONE 2020. 15(8): e0237572. https://doi.org/10.1371/journal.pone.0237572
- Caccialanza R, Constans T, Cotogni P , et al. Subcutaneous infusion of fluids for hydration or nutrition: a review. J Parenteral and Nutrition .2018.doi:10.1177/0148607116676593
- Nickel B, Gorski L, Kleidon T, et al. Infusion Therapy Standards of Practice. 9th Edition. J Infus Nurs. 2024. 47(1S Suppl1):S25-S26. doi:10.1097/NAN.0000000000000532
- Carr P , Rippey J, Budgeon C, et al. Insertion of peripheral intravenous cannulae in the Emergency Department: factors associated with first-time insertion success. J Vasc Access 2016; 17(2):182-190 doi:10.5301/jva.50000487
- Bittner B, Richter W, Schmidt J. Subcutaneous administration of biotherapeutics. An overview of current challenges and opportunities. BioDrugs. 2018. 32:425-440. https://doi.org/10.1007/s40259-018-0295-0
BD-112221 (10/25)






