Sort by:
true
Covera™ Vascular Covered Stent
The ONLY Covered Stent Indicated for AV Grafts and Native AV Fistula***

- Overview
- Clinical Experience
- Products & Accessories
- EIFU & Resources
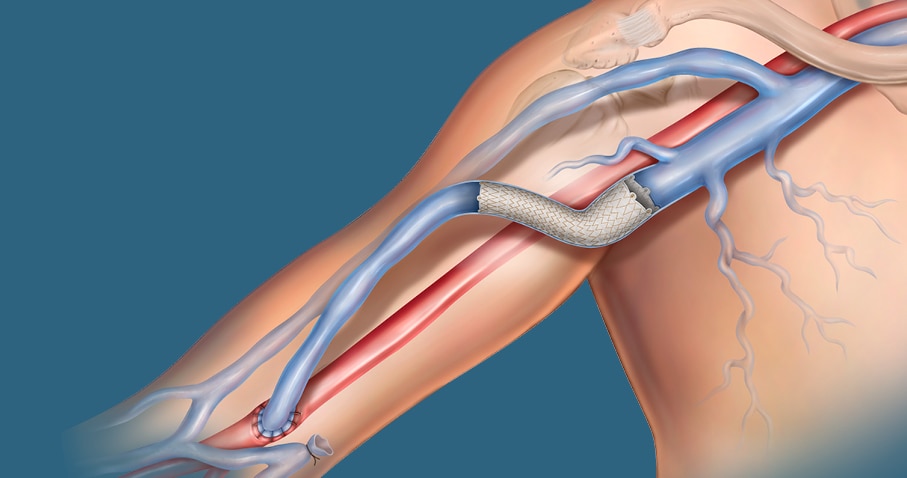
The covered stent Tailor-Made for AV patients
The Covera™ Vascular Covered Stent builds upon proven technologies from the category leader in AV access. This covered stent platform is designed to balance the flexibility and strength required to address challenging lesions from the terminal cephalic arch, to the basilic swingpoint segments, to the AV graft venous anastomosis. Flared and straight configurations allow for precise sizing and adaptation to the vessel wall, while an easy-to-use thumbwheel delivery system with two speed options provides placement control.
The Covera™ Vascular Covered Stent delivered effective results in two separate clinical trials, one for patients dialyzing with AV grafts and one for patients dialyzing with AV fistulas.1
For radial strength and flexibility
Unique, flexible base stent architecture designed to conform to native vessel in challenging AV anatomy.
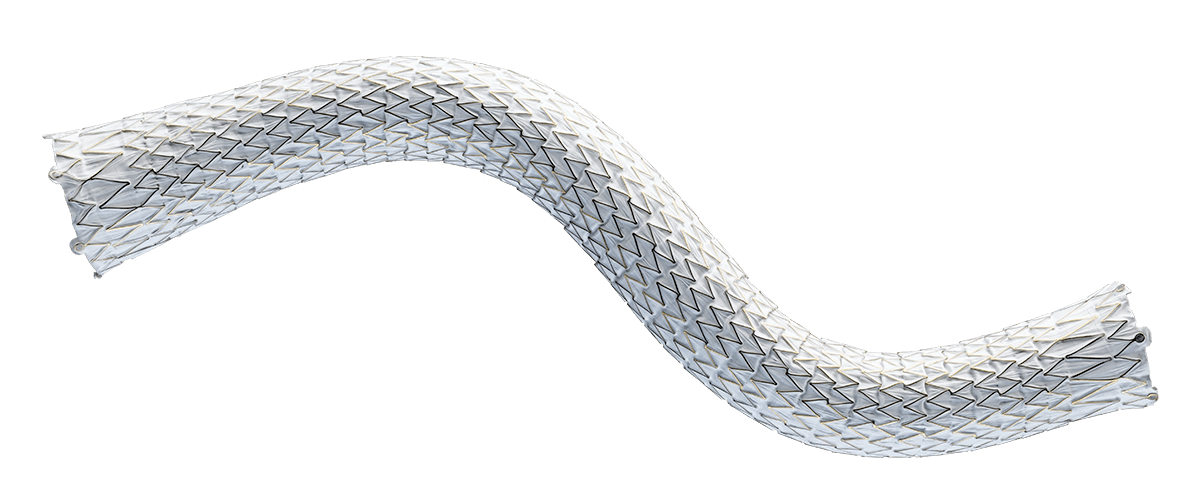
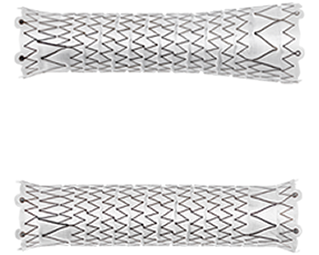
Straight and Flared configurations for optimized hemodynamic flow at the venous anastomosis
- Straight configurations are intended for use in anatomies where the diameter of the outflow vessel is equal to or smaller than that of the inflow vessel
- Flared configuration is intended for use in anatomies where the diameter of the outflow vessel is larger than that of the inflow vessel

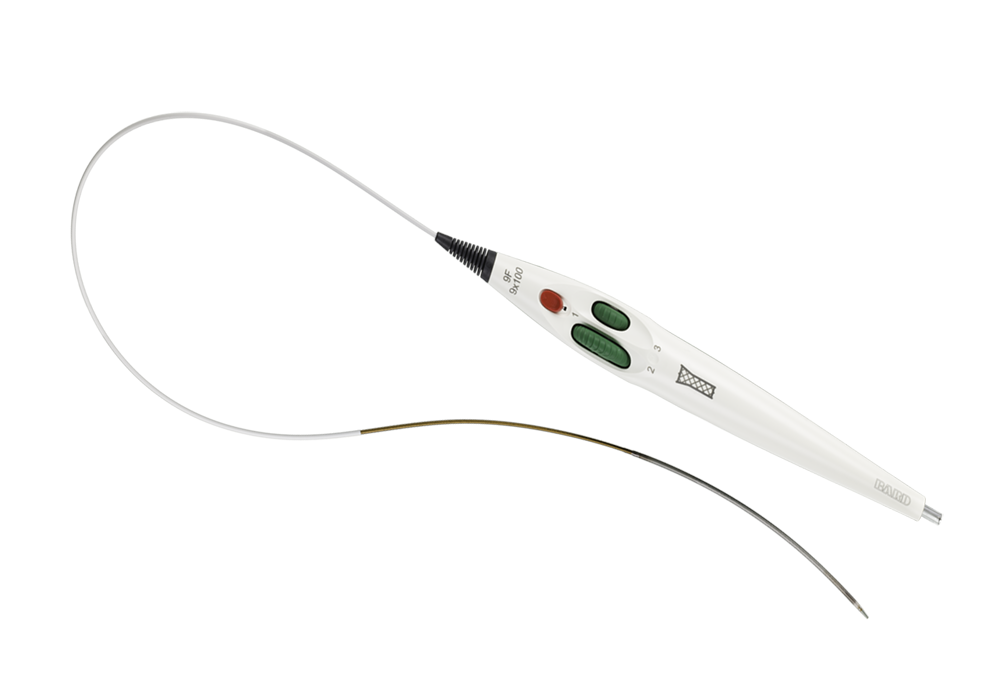
Atraumatic tip designed for ease of insertion and removal at the access site
Facilitates accurate placement control
Intuitive triaxial delivery system designed for precise placement and to facilitate optimal lesion coverage
*** In the U.S. as of Jan. 2023
1 Dolmatch B, Waheed U, Balamuthusamy S, Hoggard J, Settlage R; AVeVA Trial Investigators. Prospective, Multicenter Clinical Study of the Covera Vascular Covered Stent in the Treatment of Stenosis at the Graft-Vein Anastomosis of Dysfunctional Hemodialysis Access Grafts. J Vasc Interv Radiol. 2022;33(5):479-488.e3. doi:10.1016/j. jvir.2022.02.008. AVeNEW Clinical Studies data on file. At 6 months in AVeVA target lesion primary patency (TLPP) was 70.3% (proportional analysis). At 6 months in AVeNEW, TLPP was 78.7% for Covera™ Vascular Covered Stent vs. 47.9% for PTA alone, P < .001. 130 of the 142 (91.5%) subjects randomized to the Covera™ Vascular Stent group and 123 of the 138 (89.1%) randomized to PTA completed their 6-month follow-up. TLPP defined as the interval following the index intervention until the next clinically-driven reintervention at or adjacent to the original treatment site or until the extremity was abandoned for permanent access. In AVeNEW, TLPP at 6 Months – Subgroup Analysis is provided as observational data without P values. In AVeNEW, patients who received the Covera™ Vascular Covered Stent had 103 reinterventions involving a new lesion compared to 72 reinterventions in the PTA only group at 24 months. At 30 days, freedom from primary safety events was 96.4% in AVeVa and 95.0% (Covera™ Vascular Covered Stent) vs. 96.4% (PTA alone) in AVeNEW (P < .0022). Freedom from primary safety events was defined as freedom from an adverse event involving the access circuit resulting in additional intervention, surgery, hospitalization, or death.
Please consult Instructions for Use for product indications for use, contraindications, warnings, precautions, complications, adverse events and detailed safety information.
BD-23522v2
The Covera™ Vascular Covered Stent has been designed to address challenging lesions within in the AV access circuit – including the terminal cephalic arch, the basilic swingpoint segments, and the vein-graft anastomosis.
The Covera™ Vascular Covered Stent delivered effective results in two separate clinical trials, one for patients dialyzing with AV grafts and one for patients dialyzing with AV fistulas, both of which demonstrated the benefits of the stent’s innovative design.1
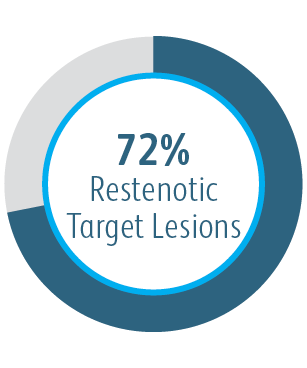
AVeVA was a prospective, non-randomized, single arm, multi-center study of the Covera™ Vascular Covered Stent used to treat stenoses at the anastomosis of an arteriovenous graft and outflow vein. 110 patients were treated with the Covera™ Vascular Covered Stent at 14 investigational sites in the US
The AVeVA Clinical Study demonstrated that the Covera™ Vascular Covered Stent is effective in the treatment of stenoses at the vein-graft anastomosis of patients dialyzing with an AV graft.
| Study Design | Prospective, Non-Randomized, Multi-Center, Single-Arm |
| Objective | To assess the safety and effectiveness of the Covera™ Vascular Covered Stent for the treatment of stenotic lesions at the graft-vein anastomosis of hemodialysis patients dialyzing with an AV graft |
| Status | 24-month follow-up completed |
| Number of Patients/Sites | 110 patients were treated with the Covera™ Vascular Covered Stent at 14 investigational sites in the US |
| Primary Effectiveness Endpoint | Target Lesion Primary Patency (TLPP) - 6 months |
| Primary Safety Endpoint | Freedom from an adverse event involving the access circuit resulting in additional intervention, surgery, hospitalization, or death through 30 days |
| Follow-Up | 30 & 90 days; 6, 12, 18 & 24 months |
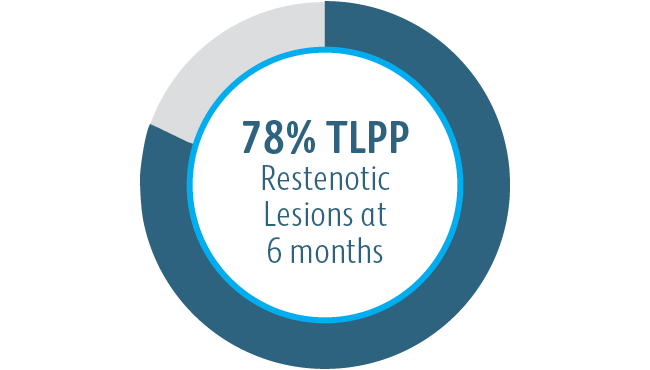
6-Month Target Lesion Primary Patency (TLPP) After Treatment of AV Graft Anastomotic Stenoses with BD Stent Grafts
This chart is for educational purposes only and not for comparison. Differences in study design may impact results. Reference full manuscripts for complete study design details.
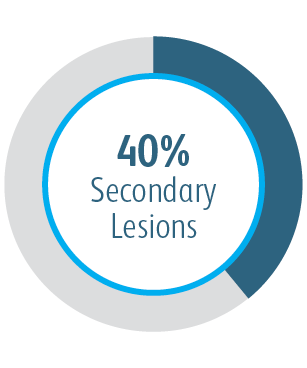
In the AVeVA Clinical Study, the Covera™ Vascular Covered Stent was studied in a challenging patient cohort.
| Study Design | Prospective, Multi-Center, Randomized, Concurrently-Controlled |
| Objective | To assess the safety and effectiveness of the Covera™ Vascular Covered Stent for the treatment of stenotic lesions in the upper extremity venous outflow of the AV access circuit of hemodialysis patients dialyzing with an AV fistula |
| Status | 24-month follow-up completed |
| Number of Patients/Sites | 280 randomized subjects in 24 investigational sites (US, EU, & ANZ) |
| Primary Effectiveness Endpoint | Target Lesion Primary Patency (TLPP) - 6 months |
| Primary Safety Endpoint | Freedom from any serious protocol-defined safety event(s) involving the AV access circuit through 30 days |
| Follow-Up | At hospital discharge, 30 & 90 days; 6, 12, 18 & 24 months |
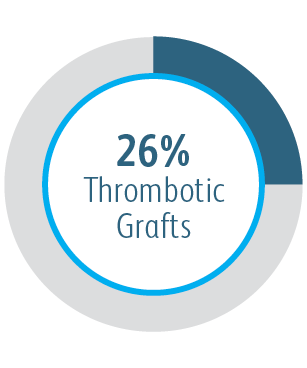
In the AVeNEW Clinical Study, the Covera™ Vascular Covered Stent was used in the treatment of a challenging patient population with difficult lesion characteristics.
The Covera™ Vascular Covered Stent was superior to the PTA control at 6 & 12 months with respect to TLPP for treatment of stenoses in the venous outflow of patients dialyzing with an arteriovenous fistula.
All subgroups showed benefit at 6 months
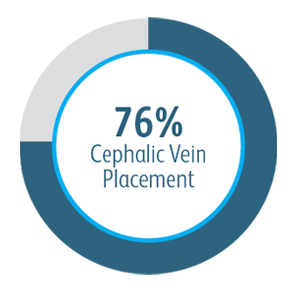
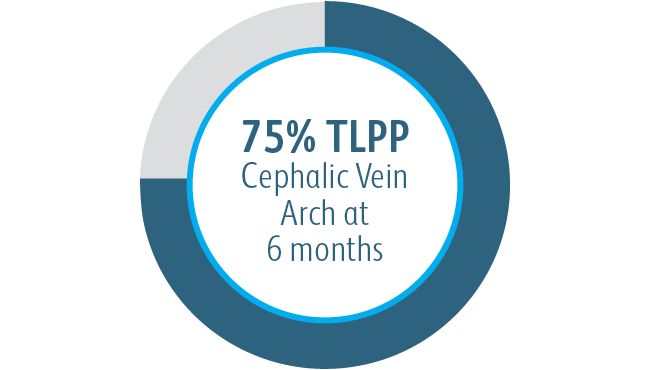
In all target lesion location subgroups analyzed, the Covera™ Vascular Covered Stent demonstrated greater target lesion primary patency compared to PTA alone, including those treated at the cephalic vein arch.
AVeNEW clinical study additional endpoints
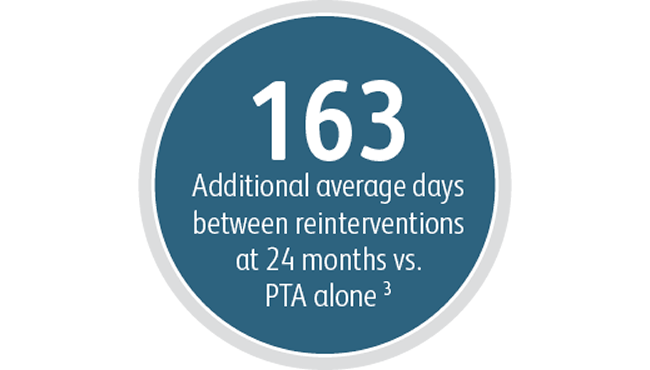
Beyond the benefits in target lesion primary patency, the Covera™ Vascular Covered Stent demonstrated a high degree of Acute Technical Success and extended the average time between interventions at the target lesion compared to PTA alone.
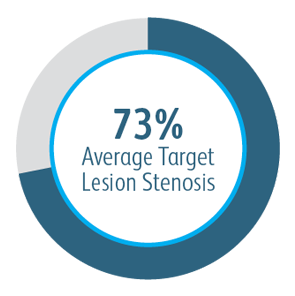
In the AVeNEW clinical study, 77 patients with stenoses in the cephalic vein arch were treated with the
Covera™ Vascular Covered Stent.
This case example demonstrates the baseline angiography showing vessel narrowing and the acute technical success with restoration of the vessel and conformability of the covered stent.
* Haskal ZJ, Trerotola S, Dolmatch B, et al. Stent graft versus balloon angioplasty for failing dialysis-access grafts. N Engl J Med. 2010 Feb 11;362(6):494-503.
** Haskal ZJ, Saad TF, Hoggard JG, et al. Prospective, randomized, concurrently-controlled study of a stent graft versus balloon angioplasty for treatment of arteriovenous access graft stenosis: 2-year results of the RENOVA study. J Vasc Interv Radiol. 2016 Aug;27(8):1105-1114.e3.
***In U.S. as of Nov. 2022
1 Dolmatch B, Waheed U, Balamuthusamy S, Hoggard J, Settlage R; AVeVA Trial Investigators. Prospective, multicenter clinical study of the Covera Vascular Covered Stent in the treatment of stenosis at the graft-vein anastomosis of dysfunctional hemodialysis access grafts. J Vasc Interv Radiol. 2022;33(5):479-488.e3. doi:10.1016/j. jvir.2022.02.008. AVeNEW Clinical Studies data on file. At 6 months in AVeVA, target lesion primary patency (TLPP) was 70.3% (proportional analysis). At 6 months in AVeNEW, TLPP was 78.7% for Covera™ Vascular Covered Stent vs. 47.9% for PTA alone, P < .001. 130 of the 142 (91.5%) subjects randomized to the Covera™ Vascular Stent group and 123 of the 138 (89.1%) randomized to PTA completed their 6-month follow-up. TLPP defined as the interval following the index intervention until the next clinically-driven reintervention at or adjacent to the original treatment site or until the extremity was abandoned for permanent access. In AVeNEW, TLPP at 6 Months – Subgroup Analysis is provided as observational data without P values. In AVeNEW, patients who received the Covera™ Vascular Covered Stent had 103 reinterventions involving a new lesion compared to 72 reinterventions in the PTA only group at 24 months. At 30 days, freedom from primary safety events was 96.4% in AVeVa and 95.0% (Covera™ Vascular Covered Stent) vs. 96.4% (PTA alone) in AVeNEW (P < .0022). Freedom from primary safety events was defined as freedom from an adverse event involving the access circuit resulting in additional intervention, surgery, hospitalization, or death.
2 Acute Technical Success was defined as successful deployment, based on the operator’s opinion, of the implant to the intended location assessed at the time of the index procedure. AVeNEW Clinical Study. Data on File. Bard Peripheral Vascular Inc., Tempe, AZ.
3 Index of Patency Function – Target Lesion (IPF-T) is defined as the time from the index study procedure to study completion or complete access abandonment divided by the number of visits for a reintervention performed at the target lesion in order to maintain vascular access for hemodialysis. Mean IPF-T of 380.40 days with Covera™ Vascular Covered Stent vs. 217.57 days with PTA alone at 24 months. AVeNEW Clinical Study. Data on File. Bard Peripheral Vascular Inc., Tempe, AZ.
Please consult Instructions for Use for product indications for use, contraindications, warnings, precautions, complications, adverse events and detailed safety information.
BD-23522v2
true
Literature
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.
Learn more
Events
We support the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
Learn more
Training
The BD Learning Academy offers a centralized repository of BD product training, education, and the ability to connect courses to your LMS.
Learn More
Case Studies
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
Learn more
true
true