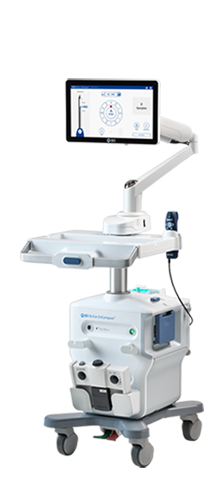
Indications for Use
The EnCor EnCompass™ Breast Biopsy and Tissue Removal System is indicated to acquire breast tissue for histologic examination with partial or complete removal of the abnormality.
Contraindications
1. This device is not intended for use except as indicated.
2. The EnCor EnCompass™ Breast Biopsy and Tissue Removal System is contraindicated for those patients where, in the physician’s judgment, there is an increased risk of complications associated with percutaneous removal of tissue samples.
Warnings
Attention: Histologic findings require correlation with imaging and clinical findings to determine concordance. If there is discordance between imaging, clinical findings, and/or pathology, further histological evaluation and potential, additional diagnostic or excisional procedures are still needed. Whenever breast tissue is removed, histological evaluation of the tissue should be performed per standard of care. Clinical and imaging surveillance should be conducted in accordance with established clinical practice guidelines and institutional protocols.
1. The EnCor EnCompass™ Breast Biopsy and Tissue Removal System Console is MR Unsafe and may not be placed in an MRI suite. Place the console outside of the MRI suite and use the appropriate MRI accessories when performing a biopsy under MRI guidance. Refer to the MRI Information section for information about the conditions for MR safety and setting up the system in MRI procedure rooms.
2.The EnCor EnCompass™ Breast Biopsy and Tissue Removal System must be properly grounded to ensure patient safety. A medical grade power cable with AC plug, available separately, provides power to the system. Do not connect the power cable to extension cords or to three-prong to two-prong adapters. To avoid the risk of electric shock, this equipment must only be connected to supply mains with protective earth.
3. To minimize interference with other equipment, cables should be positioned in such a manner to prevent contact with other cables.
4. Use of accessories not compatible with the EnCor EnCompass™ Breast Biopsy and Tissue Removal System may create potentially hazardous conditions.
5. No modification of this equipment is allowed. Do not remove the EnCor EnCompass™ Breast Biopsy and Tissue Removal System housing. Removal of the housing may cause electrical shock. Modifications could void the user’s authority to operate this device under 47 CFR § 15.21.
6. The EnCor EnCompass™ Breast Biopsy and Tissue Removal System is not classified as an AP or APG classified device. The system is not suitable for use in the presence of flammable anesthetic.
7. Do not use in the presence of infection.
8. Use of this equipment adjacent to or stacked with other equipment should be avoided because it could result in improper operation. If such use is necessary, this equipment and the other equipment should be observed to verify that they are operating normally.
9. Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm (12 inches) to any part of the EnCor EnCompass™ Breast Biopsy and Tissue Removal System, including cables specified by the manufacturer. Otherwise, degradation of the performance of this equipment could result.
10. Use of accessories, transducers and cables other than those specified or provided by BD could result in increased electromagnetic emissions or decreased electromagnetic immunity of this equipment and result in improper operation.
11. As per routine biopsy procedure, it may be necessary to cut tissue that adheres to the probe during removal from the breast.
12. Do not use the EnCor EnCompass™ Breast Biopsy and Tissue Removal System in an aircraft.
13. This device is not recommended for use in patients with breast implants.
Precautions
1. The EnCor EnCompass™ Breast Biopsy and Tissue Removal System should only be used by a physician trained in its indicated use, limitations, and in possible complications of percutaneous needle techniques.
2. Carefully inspect the device including the console body, touchscreen monitor, monitor arm, device cables and wheels prior to use to verify that the device has not been damaged. Do not use if product damage is evident.
3. Locate the EnCor EnCompass™ Breast Biopsy and Tissue Removal System as far as possible from other electronic equipment to minimize interference or degradation of performance of the EnCor EnCompass™ Breast Biopsy and Tissue Removal System.
4. Inspect accessories and cords for breaks, cracks, nicks, or other damage before every use. If damaged, do not use. Failure to observe this precaution may result in injury or electrical shock to the patient or to the operator.
5. Inspect tubing connections to ensure proper vacuum levels are achieved and maintained during use.
6. The vacuum canister is designed for multiple uses and may remain installed in the console. Inspect the vacuum canister to ensure the canister liner bag lid is secure and that no damage has occurred during shipping or installation. Please note, a heavily scratched canister can break during use. It is recommended that users replace the vacuum canister once every twelve (12) months, or on an as needed basis, to account for wear and tear.
7. Do not leave the EnCor EnCompass™ Breast Biopsy and Tissue Removal System powered on overnight.
8. Connect the power cable to a hospital grade wall outlet having the correct voltage or product damage may result.
9. Patients who may have a bleeding disorder or who are receiving anticoagulant therapy may be at increased risk of complications.
10. As with any biopsy instrument, there is a potential for infection.
11. All breast biopsies should be performed under imaging guidance to confirm the EnCor EnCompass™ Probe position relative to the target region to be sampled and to help mitigate the occurrence of a false negative biopsy.
12. When performing a biopsy with EnCor EnCompass™ Probes, the orientation of the sample notch is dictated by the image guidance selected. Prior to initiating the procedure, confirm that the sample notch orientation is correct for the image guidance being used.
13. Ensure that the EnCor EnCompass™ Breast Biopsy and Tissue Removal System is positioned in such a way that the power cable and retainer are accessible. In the event that the system power button is inoperable, remove cord to shut off system power.
14. The emissions characteristics of this equipment make it suitable for use in industrial areas and hospitals (CISPR 11 class A). If it is used in a residential environment (for which CISPR 11 class B is normally required) this equipment might not offer adequate protection to radio-frequency communication services. The user might need to take mitigation measures, such as relocating or re-orienting the equipment.
15. The product should only be used by a physician trained in its indicated use, limitations, and possible complications of percutaneous needle techniques
Potential Complications
Potential complications include, but are not limited to, hematoma, hemorrhage, hemothorax, pneumothorax, surgical intervention, infection, adjacent tissue injury, pain, allergic reaction, and tissue adherence to the biopsy probe during removal from the breast.
EnCor EnCompass™ Breast Biopsy and Tissue Removal System: Probe & Canister Liner Bag
Indications for Use
The EnCor EnCompass™ Breast Biopsy and Tissue Removal System is indicated to acquire breast tissue for histologic examination with partial or complete removal of the abnormality.
Contraindications
1. This device is not intended for use except as indicated.
2. The EnCor EnCompass™ Breast Biopsy and Tissue Removal System is contraindicated for those patients where, in the physician’s judgment, there is an increased risk of complications associated with percutaneous removal of tissue samples.
Warnings
Attention: Histologic findings require correlation with imaging and clinical findings to determine concordance. If there is discordance between imaging, clinical findings, and/or pathology, further histological evaluation and potential, additional diagnostic or excisional procedures are still needed. Whenever breast tissue is removed, histological evaluation of the tissue should be performed per standard of care. Clinical and imaging surveillance should be conducted in accordance with established clinical practice guidelines and institutional protocols.
• Care must be taken when positioning the device trocar tip near the chest wall or skin margin.
• Do not resterilize. After resterilization, the sterility of the product is not guaranteed because of an indeterminable degree of potential pyrogenic or microbial contamination which may lead to infectious complications. Cleaning, reprocessing and/or resterilization of the present medical device increases the probability that the device will malfunction due to potential adverse effects on components that are influenced by thermal and/or mechanical changes.
• Do not reuse. This device has been designed for single use only. Reusing this medical device bears the risk of cross-patient contamination as medical devices – particularly those with long and small lumina, joints, and/or crevices between components – are difficult or impossible to clean once body fluids or tissues with potential pyrogenic or microbial contamination have had contact with the medical device for an indeterminable period of time. The residue of biological material can promote the contamination of the device with pyrogens or microorganisms which may lead to infectious complications.
• Use of accessories not compatible with the EnCor EnCompass™ Breast Biopsy and Tissue Removal System may create potentially hazardous conditions.
• After use, this product may be a potential biohazard. Handle and dispose of in accordance with acceptable medical practice and applicable local, state, and federal laws and regulations. Dispose of sharps in designated sharps disposal containers; these are single-use devices and therefore should be disposed of appropriately, not recapped or resterilized.
• This device is not recommended for use in patients with breast implants.
• Do not use in the presence of infection.
• As per routine biopsy procedure, it may be necessary to cut tissue that adheres to the probe during removal from the breast.
Precautions
1. A scalpel should be used to incise the skin prior to inserting the trocar tip.
2. The product should only be used by a physician trained in its indicated use, limitations, and possible complications of percutaneous needle techniques.
3. Do not use the EnCor EnCompass™ Probe if the product sterile barrier system or its packaging is compromised.
4. Inspect the packaging to ensure that packaging integrity has not been compromised. The EnCor EnCompass™ Probe is sterile unless the seal is broken.
5. Carefully inspect the product prior to use to verify that the device has not been damaged. Do not use if product damage is evident and/or needle is bent.
Potential Complications
Potential complications may include, but are not limited to, hematoma, hemorrhage, hemothorax, pneumothorax, surgical intervention, infection, adjacent tissue injury, pain, allergic reaction, and tissue adherence to the biopsy probe during removal from the breast.
EnCor EnCompass™ Breast Biopsy and Tissue Removal System: Stereotactic Adapter
Indications for Use
The EnCor EnCompass™ Breast Biopsy and Tissue Removal System is indicated to acquire breast tissue for histologic examination with partial or complete removal of the abnormality.
Contraindications
1. This device is not intended for use except as indicated.
2. The EnCor EnCompass™ Breast Biopsy and Tissue Removal System is contraindicated for those patients where, in the physician’s judgment, there is an increased risk of complications associated with percutaneous removal of tissue samples.
Warnings
Attention: Histologic findings require correlation with imaging and clinical findings to determine concordance. If there is discordance between imaging, clinical findings, and/or pathology, further histological evaluation and potential, additional diagnostic or excisional procedures are still needed. Whenever breast tissue is removed, histological evaluation of the tissue should be performed per standard of care. Clinical and imaging surveillance should be conducted in accordance with established clinical practice guidelines and institutional protocols.
• Care must be taken to set up the EnCor EnCompass™ Stereotactic Adapter per the applicable biopsy table Instructions for Use to avoid chest wall injury.
• Use of accessories not compatible with the EnCor EnCompass™ Breast Biopsy and Tissue Removal System may create potentially hazardous conditions.
• Do not use in the presence of infection.
• No modification of this equipment is allowed.
• Exercise care when handling the EnCor EnCompass™ Stereotactic Adapter while the EnCor EnCompass™ Driver is in the primed position.
• Verify secure attachment of the EnCor EnCompass™ Probe to the EnCor EnCompass™ Stereotactic Adapter mounting pins to avoid misalignment of the EnCor EnCompass™ Probe tip or detachment of the EnCor EnCompass™ Driver.
• The EnCor EnCompass™ Stereotactic Adapter should only be used in conjunction with the appropriate needle guide.
• As per routine biopsy procedure, it may be necessary to cut tissue that adheres to the probe during removal from the breast.
Precautions
• This device should only be used by physicians trained in percutaneous biopsy procedures.
• Carefully inspect the device prior to use to verify that the device has not been damaged. Do not use if product damage is evident.
• Sterilization of the device is not necessary under normal use conditions.
Potential Complications
Potential complications may include, but are not limited to, hematoma, hemorrhage, hemothorax, pneumothorax, surgical intervention, infection, adjacent tissue injury, pain, allergic reaction, and tissue adherence to the biopsy probe during removal from the breast.
EnCor EnCompass™ Breast Biopsy and Tissue Removal System: VisiLoc™ MRI Introducer Set
Indications for Use
The EnCor EnCompass™ VisiLoc™ Introducer Set is indicated for use to penetrate the breast under image guidance and provide a passageway through which a diagnostic biopsy of the breast may be performed.
Contraindications
This device is not intended for use except as indicated.
Warnings
• Care must be taken when positioning the device trocar tip near the chest wall or skin margin.
• This device is not recommended for use in patients with breast implants.
• Do not use in the presence of infection.
• This device has been designed for single use only. Reusing this medical device bears the risk of cross-patient contamination as medical devices – particularly those with long and small lumina, joints, and/or crevices between components – are difficult or impossible to clean once body fluids or tissues with potential pyrogenic or microbial contamination have had contact with the medical device for an indeterminable period of time. The residue of biological material can promote the contamination of the device with pyrogens or microorganisms which may lead to infectious complications.
• Do not resterilize. After resterilization, the sterility of the product is not guaranteed because of an indeterminable degree of potential pyrogenic or microbial contamination which may lead to infectious complications. Cleaning, reprocessing and/or resterilization of the present medical device increases the probability that the device will malfunction due to potential adverse effects on components that are influenced by thermal and/or mechanical changes.
• After use, this product may be a potential biohazard. Handle and dispose of in accordance with acceptable medical practice and applicable local, state, and federal laws and regulations. Dispose of sharps in designated sharps disposal containers; these are single-use devices and therefore should be disposed appropriately, not recapped or resterilized.
• Keep dry. Keep away from sunlight.
Precautions
• Handle carefully as Trocar tip is sharp.
• A scalpel should be used to incise the skin prior to inserting the Trocar tip.
• The EnCor EnCompass™ Breast Biopsy and Tissue Removal System and accessories should only be used by a physician trained in its indicated use, limitations, and possible complications of percutaneous needle techniques.
• Carefully inspect the device prior to use to verify that device has not been damaged. Do not use if product damage is evident and/or needle is bent.
• Exercise caution in proximity to the magnet by maintaining control of the VisiLoc™ Trocar and EnCor EnCompass™ Probe, which may accelerate in a strong magnetic field.
• Verify that the VisiLoc™ Cannula does not move when inserting or removing the VisiLoc™ Trocar/VisiLoc™ Obturator.
• Inspect the packaging to ensure that package integrity has not been compromised. Do not use if the product sterile barrier system or its packaging is compromised. The device is sterile unless the seal is broken.
• The EnCor EnCompass™ VisiLoc™ MRI Introducer Set may be used under the following conditions:
• Static magnetic field of 3 or 1.5 Tesla
• Maximum spatial field gradient of 800 Gauss/cm or less for the VisiLoc™ Trocar and 12,000 Gauss/cm or less for the VisiLoc™ Obturator. The EnCor EnCompass™ Breast Biopsy and Tissue Removal System Console (ENCPConsole) must be kept outside the room housing the magnet.
Potential Complications
Potential complications may include, but are not limited to, hemorrhage, infection, tissue injury, pain, surgical intervention, pneumothorax, foreign body in the patient and inflammation.
Please consult product labels and inserts for complete indications, contraindications, hazards, warnings, precautions and instructions for use.
BD, the BD logo, EnCor EnCompass and VisiLoc are trademarks of Becton, Dickinson and Company or its affiliates. © 2026 BD. All rights reserved. BD-167469