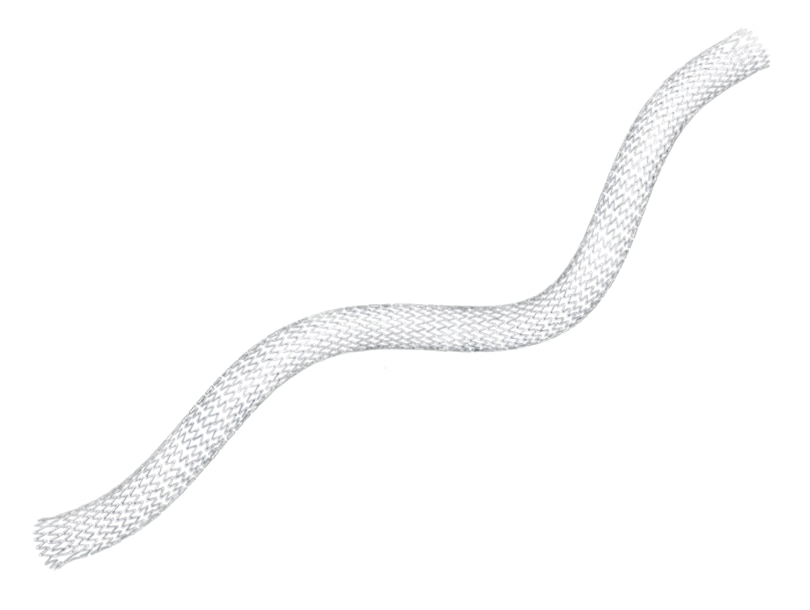
The LifeStent™ Vascular Stent System has achieved lasting results over the long term, with sustained effectiveness up to three years, and treatment superiority over balloon angioplasty. As the only commercially available bare metal stent FDA-approved for the superficial femoral and popliteal arteries, the LifeStent™ Vascular Stent has a history of proven performance. With its unique helical design, it is engineered for bending, compression, and torsion with dynamic vessel conformability. The LifeStent™ Vascular Stent Systems, in varying sizes, have been studied in more than ten clinical trials in the United States and globally.
The LifeStent™ Vascular Stent is a peripheral stent intended to improve luminal diameter in the treatment of symptomatic de-novo or restenotic lesions up to 240mm in length in the native superficial femoral artery (SFA) and popliteal artery with reference vessel diameters ranging from 4.0 – 6.5mm. The LifeStent™ Vascular Stent is the only FDA-approved stent for the SFA and full popliteal artery. The LifeStent™ Vascular Stent is available in 5 mm, 6 mm, and 7 mm diameters; and 20 mm to 170 mm in length.
Additionally, the LifeStent™ Solo™ Vascular Stent is available in 6 mm and 7 mm diameters and 200 mm in length.