The OptiFix™ Absorbable Fixation System has been engineered inside and out to provide surgeon confidence and secure fixation in a reliable, easy to use, and ergonomic design.


- Overview
- Products & Accessories
- EIFU & Resources
Challenges and opportunities in absorbable fixation
Absorbable Fixation devices can provide important benefits and lead to repeatable outcomes. However, Absorbable Fixation devices that facilitate soft tissue repair may also be associated with certain challenges, such as:
- Excessive deployment force may cause tissue trauma and bleeding1
- Fasteners may be difficult to see laparoscopically potentially leading to the placement of unnecessary fasteners
- Device may be uncomfortable or awkward to deploy in smaller sized hands and may be sensitive to surgeon technique
The OptiFix™ Absorbable Fastener is made from Poly(D, L-Lactide) and is designed for optimal performance. Fastener features include:
Smooth Fastener Head
• Minimizes the potential for tissue attachment1
• Ensures mesh is securely fixated
Hollow Core Design
• Allows tissue ingrowth through the fastener1
Angled Tip
• Easily penetrates mesh and tissue1
Stabilizers
• Enhances tissue holding strength
• Prevents the fastener from backing out
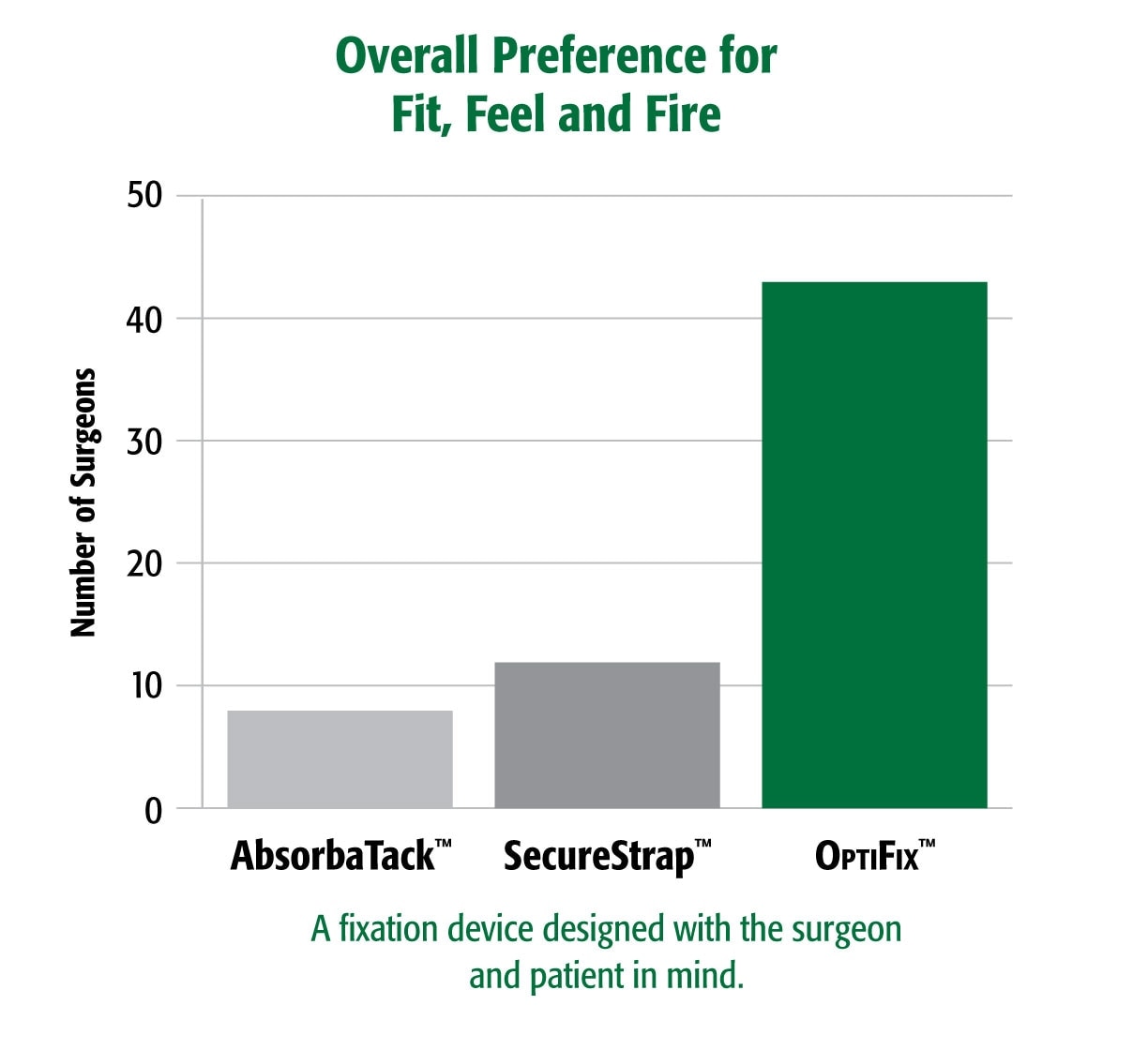
Surgeon Feedback4 : Surgeons prefer the handling of OptiFix™ versus other fixation devices In a blind preference test assessing surgeons’ preference of device comfort, trigger force, and overall fit, feel and fire, OptiFix™ was preferred nearly 4x more frequently than the next closest competitor.
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.
We support the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
The BD Learning Academy offers a centralized repository of BD product training, education, and the ability to connect courses to your LMS.
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
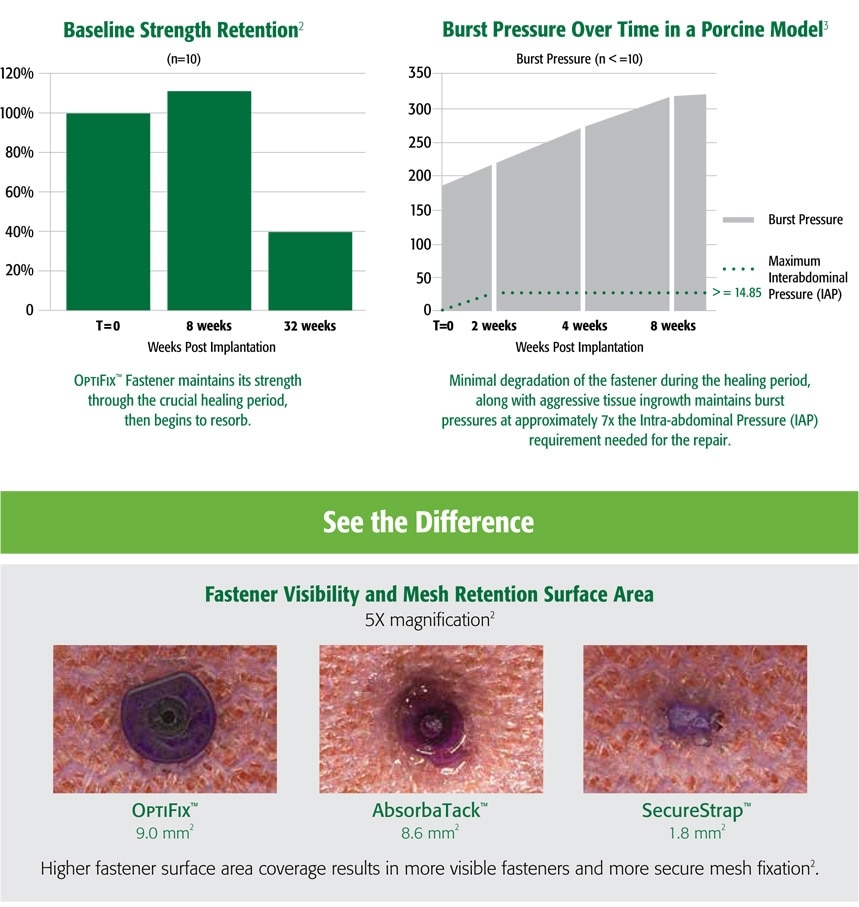
- Preclinical data on file. Results may not correlate to clinical outcomes.
- C. R. Bard Inc., bench data on file.
- Preclinical data on file. Results may not correlate to clinical outcomes.
- Survey of surgeons attending an international surgical conference. Data on file.
INDICATIONS
The OptiFix™ Absorbable Fixation System is indicated for the approximation of soft tissue and fixation of surgical mesh to tissues during open or laparoscopic surgical procedures, such as hernia repair.
CONTRAINDICATIONS
1. This device is not intended for use except as indicated.
2. Do not use this device where hemostasis cannot be verified visually after application.
3. Contraindications associated with open and laparoscopic surgical procedures relative to mesh fixation apply, including but not limited to:
- Fixation of vascular or neural structures
- Fixation of bone and cartilage
- Situations with insufficient ingrowth of tissue into the mesh over time, which could result in inadequate fixation once the fastener is absorbed.
4. Carefully inspect the area in the vicinity of the tissue being fastened to avoid inadvertent penetration of underlying structures such as nerves, vessels, viscera, or bone. Use of the OptiFix™ Absorbable Fixation System in the close vicinity of such underlying structures is contraindicated. For reference, the length of the fastener below the fastener head is 6.1 mm; the fastener head is another 0.6 mm (total 6.7 mm).
5. This device should not be used in tissues that have a direct anatomic relationship to major vascular structures. This would include the deployment of fasteners in the diaphragm in the vicinity of the pericardium, aorta, or inferior vena cava during diaphragmatic hernia repair.
WARNINGS
1. The OptiFix™ Absorbable Fixation System is intended for Single Use Only – DO NOT RESTERILIZE. Reuse, reprocessing, resterilization or repackaging may compromise the structural integrity and/ or essential material and design characteristics that are critical to the overall performance of the device and may lead to device failure which may result in injury to the patient. Reuse, reprocessing, resterilization or repackaging may also create a risk of contamination of the device and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another. Contamination of the device may lead to injury, illness or death of the patient or end user. This product is supplied sterile. Inspect the packaging to be sure it is intact and undamaged prior to use.
2. Do not use beyond the expiration date on the package.
3. Prior to use, carefully examine package and product to verify neither is damaged and that all seals are intact. Do not use if the foil pouch or package is damaged or open, or if the center of the temperature indicator on the foil pouch is black.
4. As with any implant material, the presence of bacterial contamination may enhance bacterial infectivity. Accepted surgical practice must be followed with respect to drainage and closure of infected or contaminated wounds.
5. Users should be familiar with surgical procedures and techniques involving synthetic absorbable materials before employing OptiFix™ Absorbable Fixation System fasteners for wound closure, as the risk of wound dehiscence may vary with the site of application and the material used.
6. The device may not fixate through prosthetics derived from biologic material such as xenografts and allografts. Prosthetic should be evaluated for compatibility prior to use.
After use, the OptiFix™ Absorbable Fixation System may be a potential biohazard. Handle and dispose of in accordance with any local and federal laws regarding medical waste.
PRECAUTIONS
1. Please read all instructions before using the OptiFix™ Absorbable Fixation System.
2. Only persons having adequate medical training and familiarity with surgical techniques should perform surgical procedures. Consult the medical literature relative to technique, complications and hazards prior to any surgical procedure.
3. The OptiFix™ Absorbable Fixation System can be used with most 5 mm trocars. Ensure compatibility by inserting the device into the trocar prior to introduction into the patient. The OptiFix™ Absorbable Fixation System should enter and exit the trocar easily without excessive force. The use of too much force could damage the instrument.
4. Counterpressure should be applied on the target area. Avoid placing hand/finger directly over the area where fastener is being deployed to prevent injury.
5. Use caution when deploying the OptiFix™ fastener over or in proximity to underlying bone, vessels, nerves, or viscera. The intended fixation site should be assessed to ensure that while the tissue is compressed the total distance from the surface of the tissue to any underlying structures is greater than the length of the OptiFix™ fastener.
6. Avoid excessive trigger force as this may damage the device.
7. Insertion of fasteners is possible into some collagenous structures such as ligaments and tendons, but is NOT possible directly into bone or cartilage. This may damage the device and result in compromised fixation strength.
8. Care should be taken not to use excessive counterpressure as this may damage the distal tip of the device as well as the mesh and/or tissue.
9. If the device locks and cannot be separated from a fastener that has been deployed into mesh and/or tissue, place a grasper adjacent to the deployed fastener and pull to free the device. If needed, you may use laparoscopic scissors to cut below the fastener head. The remaining portion of the fastener stem left in the mesh can be removed with graspers. The device should then be discarded and a new device should be used.
10. If the fastener does not deploy properly, remove the device from the patient and test the device in gauze to ensure proper fastener deployment. Once proper fastener deployment is confirmed, the device may be reinserted into the patient.
11. The safety and effectiveness of the OptiFix™ Absorbable Fixation System have not been evaluated or established in pregnant or breastfeeding women.
12. This device contains the following substance(s) defined as CMR 1B in a concentration above 0.1% weight by weight: Cobalt; CAS No. 7440-48-4; EC No. 231-158-0. Current scientific evidence supports that medical devices manufactured from stainless-steel alloys containing cobalt do not cause an increased risk of cancer or adverse reproductive effects. For more information, please consult the ECHA website: Homepage - ECHA
ADVERSE REACTIONS
Adverse reactions and potential complications associated with fixation devices such as the OptiFix™ Absorbable Fixation System may include, but are not limited to the following: hemorrhage; seroma; pain, edema and erythema at wound site; allergic reaction to Poly(D, L)-lactide; infection/septicemia; hernia recurrence/wound dehiscence; hematoma.