BD Uniject™ Auto-Disable Prefillable Injection System
BD tools for vaccine combination product developers

- Overview
- BD Expertise
- Availability
- Products & Accessories
- Resources
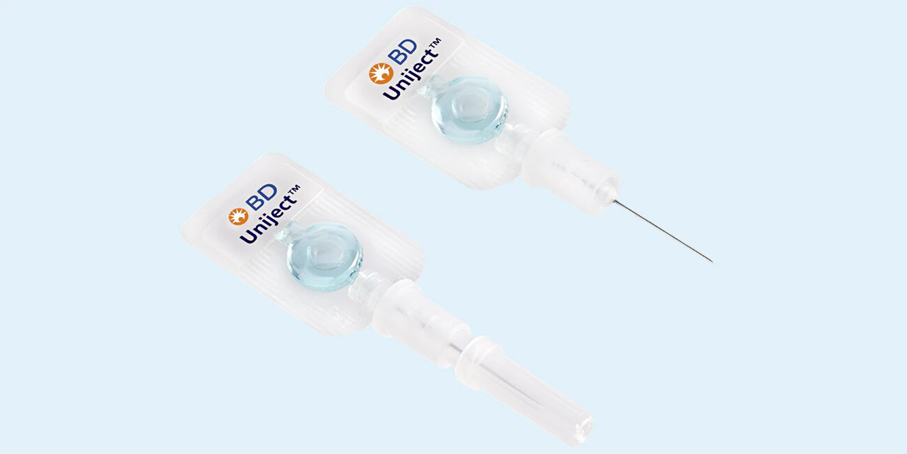
BD Uniject™ Auto-Disable Prefillable Injection System is a tool for vaccine combination product developers. Prefilled and ready to administer, the system is easy to use1 and produces less waste disposal than syringes.2, 3 It is dose-sparing with no waste of unused multi-doses. Users perceived the system as less painful4 and experienced no anxiety.5 The system comes with regulatory documentation and support, technical deployment support. Available in 0.5 mL and 1 mL volumes.
BD UnijectTM Auto-Disable Prefillable Injection System Features
- Auto-disable, all-in-one-design for simple roll-out
- Available in 0.5 mL and 1 mL volumes
- Standard needle sizes available: 23 G and 25 G needle diameter; ⅜” (10mm) – 1½” (38 mm) needle length
- Delivered in BD SCF™ Sterile, Clean and ready to Fill.
BD UnijectTM Auto-Disable Prefillable Injection System Benefits
- Pre-filled ready to administer: Vaccine protected until administration, complete content injection,5 preparation and delivery time saving. 1, 2, 6
- Easy to use:1 ease of preparation and drug administration.
- Less waste disposal than syringes. 2, 3 25% less voluminous and 62% lighter than vial.7
- Dose-sparing: no waste of unused multi-doses.
- Users perceived system as less painful4 and experienced no anxiety.5
- Comes with regulatory documentation and support, technical deployment support.
- Lavidalie et al., 2010, Pilot Introduction of Oxytocin in Uniject During Active Management of the Third Stage of Labor (AMTSL) at the Institutional Level in Guatemala, PATH. Data is from 77 providers on ease of use of oxytocin in BD UnijectTM. 92% of providers find ease of preparing the oxytocin in BD UnijectTM very easy, and 80% find ease of administering the oxytocin in BD UnjectTM very easy
- Guillermet et al., 2015, Acceptability and Feasibility of Delivering Pentavalent Vaccines in a Compact, Prefilled, Autodisable Device in Vietnam and Senegal, PLoS One. 2015 Jul 17;10(7):e0132292
- Berman et al., 2014, Pentavalent Vaccine in the UnijectTM Injection System: Health Care Waste Management Considerations, PATH
- Burke et al., 2014, Observational study of the acceptability of Sayana® Press among intramuscular DMPA users in Uganda and Senegal. In this study, 1020 women in Uganda and 242 in Senegal received Sayana® Press (SP) in BD UnijectTM. 84% of Ugandan participants and 80% of Senegalese participants said they would select SP over Intramuscular administration (DMPA IM). One of the reason for selecting SP were less pain
- Sutanto et al., Home delivery of heat-stable vaccines in Indonesia: outreach immunization with a prefilled, single-use injection device, Bulletin of the World Health Organization, 1999. In this study, 96% of the 23 midwives correctly use the Uniject device to complete the injection of contents. Of the 456 mothers who had received injections by means of the BD UnijectTM device, 94% said they had experienced no anxiety.
- Lorenson et al., 2014, Pentavalent Vaccine in the UnijectTM Injection System, A Time and Motion Study, PATH
- Childress et al. 2011, Logistics and waste management benefits of depo-subQ in Uniject, PATH 25% less voluminous and 62% lighter than DMPA vial packed with syringe and safety
Our expertise in BD Uniject™ Auto-Disable Prefillable Injection System
- 125+ million doses sold to date1
- In combination with vaccines: Pentavalent2 , Tetanus3, Hep. A3, Hep. B3
- Experience with contraceptive drugs3 and treatment of postpartum hemorrhage3
- Potential to improve access to and delivery of contraceptives, vaccines and maternal and child health medicines4
- BD analysis [Internal report]. Pont de Claix, FR: Becton, Dickinson and Company; 2021
- Pentavalent Vaccine in the Uniject™ Injection System. Lorenson et al 2014
- A Health Historical Profile, The Uniject™ device, 2005, PATH
- Childress et al., 2011, The Uniject™ injection System: Multi-country experience and evidence, PATH
- Samples available on demand
- Sample filling capacity in BD lab for testing purposes
- Filling and sealing pilot machine available for early feasibility studies
- Availability for a few million units
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.