Animal data may not correlate to outcomes in humans. Data on file.
Indications
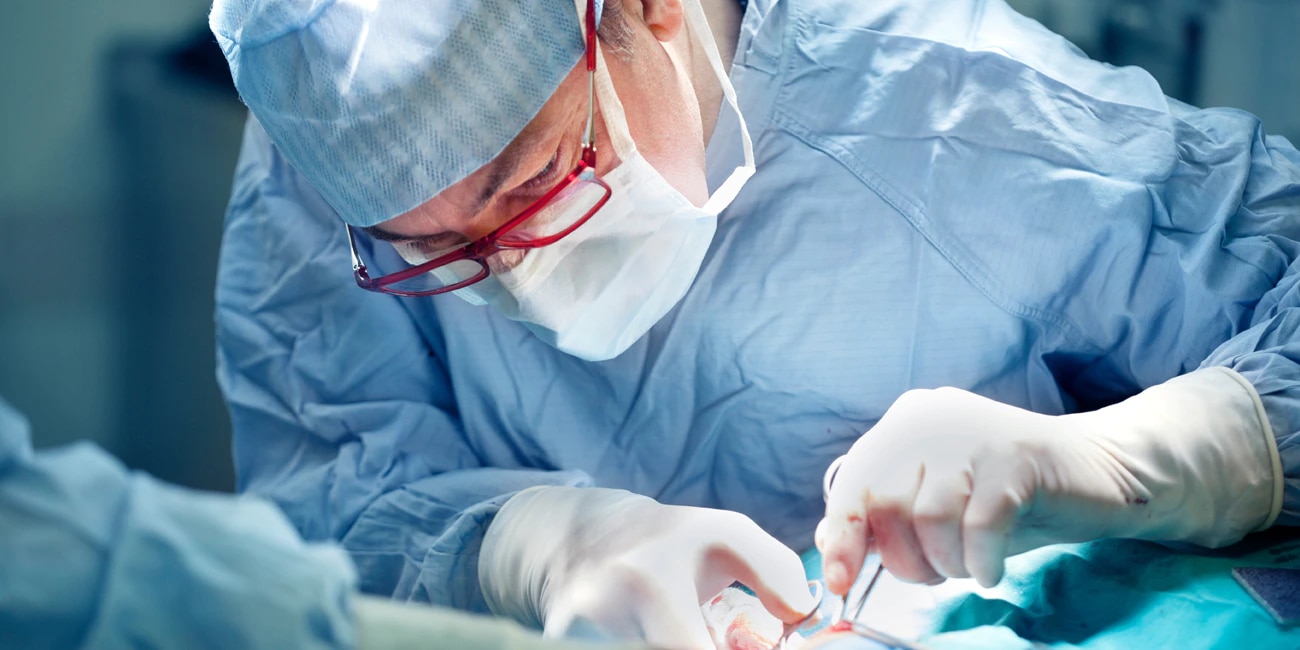
Avitene™ Microfibrillar Collagen Hemostat (MCH) and Avitene™ UltraFoam™ sponge are indicated in surgical procedures as an adjunct to hemostasis when control of bleeding by ligature or conventional procedures is ineffective or impractical.
Contraindications
- Avitene™ MCH and Avitene™ UltraFoam™ sponge should not be used in the closure of skin incisions as they may interfere with the healing of the skin edges. This is due to simple mechanical interposition of dry collagen and not to any intrinsic interference with wound healing.
- It has been reported with other collagen hemostatic agents, that by filling porosities of cancellous bone, they may significantly reduce the bond strength of methylmethacrylate adhesives. Avitene™ MCH and Avitene™ UltraFoam™ sponge should not, therefore, be employed on bone surfaces to which prosthetic materials are to be attached with methylmethacrylate adhesives.
Warnings
- Avitene™ MCH and Avitene™ UltraFoam™ are inactivated by autoclaving.
- Ethylene oxide reacts with bound hydrochloric acid to form ethylene chlorohydrin.
- These devices have been designed for single use only. Reuse, reprocessing, resterilization or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the devices and may lead to device failure which may result in injury to the patient. Reuse, reprocessing, resterilization or repackaging may also create a risk of contamination of the device and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another. Contamination of the devices may lead to injury, illness or death of the patient or end user. Opened, unused product should be discarded.
- Moistening Avitene™ MCH or wetting with saline or thrombin impairs its hemostatic efficacy. It should be used dry.
- As with any foreign substance, use of Avitene™ MCH and Avitene™ UltraFoam™ in contaminated wounds may enhance infection.
- Avitene™ UltraFoam™ sponge should not be used in instances of pumping arterial hemorrhage.
- Avitene™ UltraFoam™ should not be used where blood or other fluids have pooled, or in cases where the point of hemorrhage is submerged as it may mask an underlying source of bleeding, resulting in hematoma.
- Avitene™ UltraFoam™ sponge will not act as a tampon or plug in a bleeding site, nor will it close off an area of blood collecting behind a tampon.
- Avitene™ UltraFoam™ sponge is not intended to treat systemic coagulation disorders.
- Avitene™ MCH and Avitene™ UltraFoam™ are not for injection, intraocular or intravascular use.
Adverse Reactions
- The most serious adverse reaction reported which may be related to the use of Avitene™ MCH or other collagen products are potentiation of infection including abscess formation, hematoma, wound dehiscence and mediastinitis.
- Other reported adverse reactions possibly related are adhesion formation, allergic reaction, foreign body reaction and subgaleal seroma (report of a single case) and increased incidence of alveolalgia when used for packing of dental extraction sockets.
- Transient laryngospasm due to aspiration of dry material has been reported following use of Avitene™ MCH in tonsillectomy.
Please consult package insert for more detailed safety information and instructions for use.