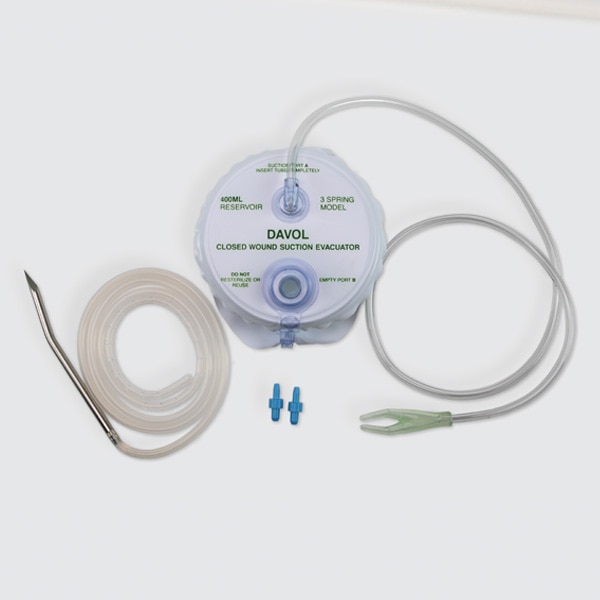
Wound drains are used to remove exudates from wound sites.

- Overview
- Channel Drains
- Closed Wound Drains
- Products & Accessories
- Resources
Warnings:
- An effective closed suction drain system requires maintenance of the system to preserve patency. The drain must not be allowed to occlude nor the reservoir to completely fill; and reservoir suction must be maintained in order for the system to function properly. Verify that the system is functioning properly. If the system is not maintained properly, surgical complications, including hematomas, may result.
- In the event of occlusion of the drain, all wound drainage via the drain ceases. Careful attention to the drain will minimize the possibility of this problem. If occlusion does occur, the drain can be aspirated by connecting suction to the reservoir outlet or temporarily disconnecting the drain from the reservoir and applying suction directly to the drain.
- If an air-tight seal between the drain and the skin where the drain emerges is not achieved, the air leak must be rectified, or the system must be converted to open drainage.
- An airtight seal between all system components (drain, adaptor and reservoir) is necessary for proper system function.
- Leaving the soft silicone elastomer drain implanted for any period of time so as to cause tissue in growth around the drain can interfere with easy removal and may affect the performance of the drain. The surgeon should monitor the patient’s rate of wound healing
- Drain perforations or channels must lie within the wound or cavity to be drained, otherwise inadequate drainage may result.
- To avoid the possibility of drain damage or breakage:
- Avoid suturing through drains.
- Drains should lie flat and in line with the skin exit areas.
- Particular care should be taken to avoid any obstacles to the drain exit path.
- Drains should be checked for free motion during closure to minimize the possibility of breakage.
- Drain removal should be done gently by hand. Drains should not be handled with pointed, toothed or sharp instruments which could cause cuts or nicks and lead to subsequent structural failure of the drain.
- Surgical removal may be necessary if drain is difficult to remove or breaks.
- Do not bypass/inactivate anti-reflux valve.
- Blood collected using the Evacuator should not be re-infused.
- DO NOT use in patients who are allergic to materials used in BD™ Drain Products.
- This is a single use device. DO NOT reuse.
- DO NOT re-sterilize.
- Trocar and evacuator are MR unsafe.
- After use, this product may be a potential biohazard. Handle and dispose of in accordance with accepted medical practice and applicable local, state, and federal laws and regulations.
Precautions
- Ensure that the wound site is dry and free of debris before closure.
- The surgeon must determine the number of drains needed for an effective drainage of the entire wound site.
- If the drain is occluded, irrigation and/or aspiration of the drain may be required.
- The quality and quantity of drained fluid must be regularly monitored and reported to the surgeon.
- Reservoir, once full, must be emptied per hospital protocols. Failure to do so will result in incomplete drainage.
- Suction must be discontinued prior to the removal of the drain.
- Before starting the Drainage Procedure, ensure that all the connections are tight and free of any obstructions within the drainage pathway.
- Di(2-ethylhexyl) phthalate (DEHP) is a plasticizer used in some polyvinyl chloride medical devices. DEHP has been shown to produce a range of adverse effects in experimental animals, notably liver toxicity and testicular atrophy. Although the toxic and carcinogenic effects of DEHP have been well established in experimental animals, the ability of this compound to produce adverse effects in humans is controversial. There is no evidence that neonates, infants, pregnant and breast-feeding women exposed to DEHP experience any related adverse effects. However, a lack of evidence of causation between DEHP-PVC and any disease or adverse effect does not mean that there are no risks.
Contraindications
- Closed Wound Suction System: Do not use for chest drainage.
Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
Please consult product labels, inserts and instructions for use for any indications, contraindications, hazards, warnings, cautions and directions for use.
BD and the BD logo are trademarks of Becton, Dickinson, and Company or its affiliates. © BD 2025. All rights reserved.
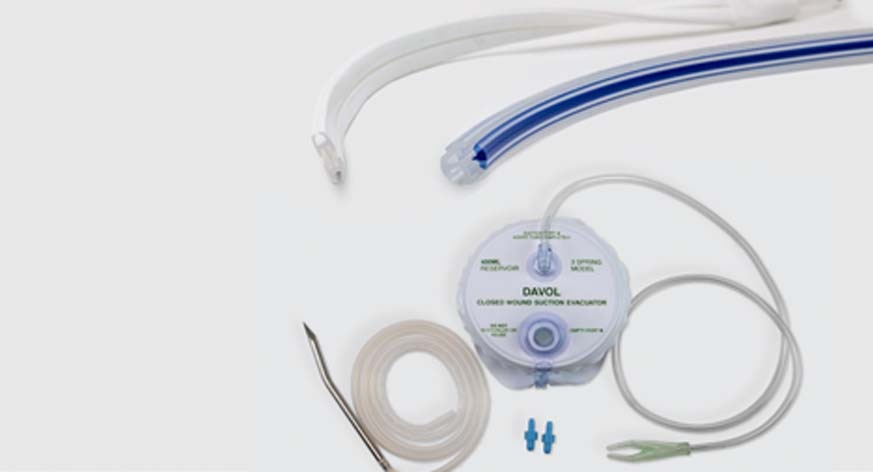
Silicone channel drains are used to remove exudates from wound sites.
- Round and Flat drains in various sizes and fluted options.
- Round drains offered as hubless
- Drains offered with a trocar option.
- Silicone. Not made with natural rubber latex.
- For use with 100cc, 200cc, and 400cc Silicone Evacuators, and 3-Spring Evacuator.
Warnings:
- An effective closed suction drain system requires maintenance of the system to preserve patency. The drain must not be allowed to occlude nor the reservoir to completely fill; and reservoir suction must be maintained in order for the system to function properly. Verify that the system is functioning properly. If the system is not maintained properly, surgical complications, including hematomas, may result.
- In the event of occlusion of the drain, all wound drainage via the drain ceases. Careful attention to the drain will minimize the possibility of this problem. If occlusion does occur, the drain can be aspirated by connecting suction to the reservoir outlet or temporarily disconnecting the drain from the reservoir and applying suction directly to the drain.
- If an air-tight seal between the drain and the skin where the drain emerges is not achieved, the air leak must be rectified, or the system must be converted to open drainage.
- An airtight seal between all system components (drain, adaptor and reservoir) is necessary for proper system function.
- Leaving the soft silicone elastomer drain implanted for any period of time so as to cause tissue in growth around the drain can interfere with easy removal and may affect the performance of the drain. The surgeon should monitor the patient’s rate of wound healing
- Drain perforations or channels must lie within the wound or cavity to be drained, otherwise inadequate drainage may result.
- To avoid the possibility of drain damage or breakage:
- Avoid suturing through drains.
- Drains should lie flat and in line with the skin exit areas.
- Particular care should be taken to avoid any obstacles to the drain exit path.
- Drains should be checked for free motion during closure to minimize the possibility of breakage.
- Drain removal should be done gently by hand. Drains should not be handled with pointed, toothed or sharp instruments which could cause cuts or nicks and lead to subsequent structural failure of the drain.
- Surgical removal may be necessary if drain is difficult to remove or breaks.
- Do not bypass/inactivate anti-reflux valve.
- Blood collected using the Evacuator should not be re-infused.
- DO NOT use in patients who are allergic to materials used in BD™ Drain Products.
- This is a single use device. DO NOT reuse.
- DO NOT re-sterilize.
- Trocar and evacuator are MR unsafe.
- After use, this product may be a potential biohazard. Handle and dispose of in accordance with accepted medical practice and applicable local, state, and federal laws and regulations.
Precautions
- Ensure that the wound site is dry and free of debris before closure.
- The surgeon must determine the number of drains needed for an effective drainage of the entire wound site.
- If the drain is occluded, irrigation and/or aspiration of the drain may be required.
- The quality and quantity of drained fluid must be regularly monitored and reported to the surgeon.
- Reservoir, once full, must be emptied per hospital protocols. Failure to do so will result in incomplete drainage.
- Suction must be discontinued prior to the removal of the drain.
- Before starting the Drainage Procedure, ensure that all the connections are tight and free of any obstructions within the drainage pathway.
- Di(2-ethylhexyl) phthalate (DEHP) is a plasticizer used in some polyvinyl chloride medical devices. DEHP has been shown to produce a range of adverse effects in experimental animals, notably liver toxicity and testicular atrophy. Although the toxic and carcinogenic effects of DEHP have been well established in experimental animals, the ability of this compound to produce adverse effects in humans is controversial. There is no evidence that neonates, infants, pregnant and breast-feeding women exposed to DEHP experience any related adverse effects. However, a lack of evidence of causation between DEHP-PVC and any disease or adverse effect does not mean that there are no risks.
Contraindications
- Closed Wound Suction System: Do not use for chest drainage.
Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
Please consult product labels, inserts and instructions for use for any indications, contraindications, hazards, warnings, cautions and directions for use.
BD and the BD logo are trademarks of Becton, Dickinson, and Company or its affiliates. © BD 2025. All rights reserved.
Closed wound drains are used to remove exudates from wound sites
- Silicone drains offered in round, double round, or flat options in various sizes, perforations, and evacuator kit options.
- Silicone flat drains offered in a hubless format.
- PVC drains offered in round and double round options in various sizes, and 400cc 3-spring evacuator kit option.
- Drains offered with a trocar option.
- Not made with natural rubber latex.
- For use with 100cc, 200cc, and 400cc Silicone Evacuators, and 3-Spring Evacuator.
Warnings:
- An effective closed suction drain system requires maintenance of the system to preserve patency. The drain must not be allowed to occlude nor the reservoir to completely fill; and reservoir suction must be maintained in order for the system to function properly. Verify that the system is functioning properly. If the system is not maintained properly, surgical complications, including hematomas, may result.
- In the event of occlusion of the drain, all wound drainage via the drain ceases. Careful attention to the drain will minimize the possibility of this problem. If occlusion does occur, the drain can be aspirated by connecting suction to the reservoir outlet or temporarily disconnecting the drain from the reservoir and applying suction directly to the drain.
- If an air-tight seal between the drain and the skin where the drain emerges is not achieved, the air leak must be rectified, or the system must be converted to open drainage.
- An airtight seal between all system components (drain, adaptor and reservoir) is necessary for proper system function.
- Leaving the soft silicone elastomer drain implanted for any period of time so as to cause tissue in growth around the drain can interfere with easy removal and may affect the performance of the drain. The surgeon should monitor the patient’s rate of wound healing
- Drain perforations or channels must lie within the wound or cavity to be drained, otherwise inadequate drainage may result.
- To avoid the possibility of drain damage or breakage:
- Avoid suturing through drains.
- Drains should lie flat and in line with the skin exit areas.
- Particular care should be taken to avoid any obstacles to the drain exit path.
- Drains should be checked for free motion during closure to minimize the possibility of breakage.
- Drain removal should be done gently by hand. Drains should not be handled with pointed, toothed or sharp instruments which could cause cuts or nicks and lead to subsequent structural failure of the drain.
- Surgical removal may be necessary if drain is difficult to remove or breaks.
- Do not bypass/inactivate anti-reflux valve.
- Blood collected using the Evacuator should not be re-infused.
- DO NOT use in patients who are allergic to materials used in BD™ Drain Products.
- This is a single use device. DO NOT reuse.
- DO NOT re-sterilize.
- Trocar and evacuator are MR unsafe.
- After use, this product may be a potential biohazard. Handle and dispose of in accordance with accepted medical practice and applicable local, state, and federal laws and regulations.
Precautions
- Ensure that the wound site is dry and free of debris before closure.
- The surgeon must determine the number of drains needed for an effective drainage of the entire wound site.
- If the drain is occluded, irrigation and/or aspiration of the drain may be required.
- The quality and quantity of drained fluid must be regularly monitored and reported to the surgeon.
- Reservoir, once full, must be emptied per hospital protocols. Failure to do so will result in incomplete drainage.
- Suction must be discontinued prior to the removal of the drain.
- Before starting the Drainage Procedure, ensure that all the connections are tight and free of any obstructions within the drainage pathway.
- Di(2-ethylhexyl) phthalate (DEHP) is a plasticizer used in some polyvinyl chloride medical devices. DEHP has been shown to produce a range of adverse effects in experimental animals, notably liver toxicity and testicular atrophy. Although the toxic and carcinogenic effects of DEHP have been well established in experimental animals, the ability of this compound to produce adverse effects in humans is controversial. There is no evidence that neonates, infants, pregnant and breast-feeding women exposed to DEHP experience any related adverse effects. However, a lack of evidence of causation between DEHP-PVC and any disease or adverse effect does not mean that there are no risks.
Contraindications
- Closed Wound Suction System: Do not use for chest drainage.
Caution: Federal (U.S.A.) law restricts this device to sale by or on the order of a physician.
Please consult product labels, inserts and instructions for use for any indications, contraindications, hazards, warnings, cautions and directions for use.
BD and the BD logo are trademarks of Becton, Dickinson, and Company or its affiliates. © BD 2025. All rights reserved.
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.
We support the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
The BD Learning Academy offers a centralized repository of BD product training, education, and the ability to connect courses to your LMS.
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.