CapSure™ provides surgeons the confidence they desire through strong and reliable fixation.


- Overview
- Products & Accessories
- EIFU & Resources
- FAQ
Traditional challenges in permanent fixation for hernia repair
Permanent fixation devices facilitate a strong long-term repair but may be associated with some challenges:
- Clinical complications from exposed metal points including adhesions to fastener3
- Difficulties securing large pore mesh may impact hernia repair outcomes3
- Deployment challenges and device reliability issues can disrupt procedures3
Bard has redefined permanent fixation
Covered
- Smooth polyetheretherketone (PEEK) cap eliminates exposed metal tip and helps minimize adhesions to the fastener*3
Strong
- Fixates into Cooper’s ligament and underlying structures, similar to ProTack™
Reliable
- Comfortable handle and easy to deploy trigger
- Consistent fastener deployment and depth of tissue purchase
- Reliable, secure fixation regardless of mesh pore size1
- Improved fixation with large pore meshes due to larger cap surface area of fastener versus ProTack™1
*Preclinical data. Results may not correlate to the performance in humans
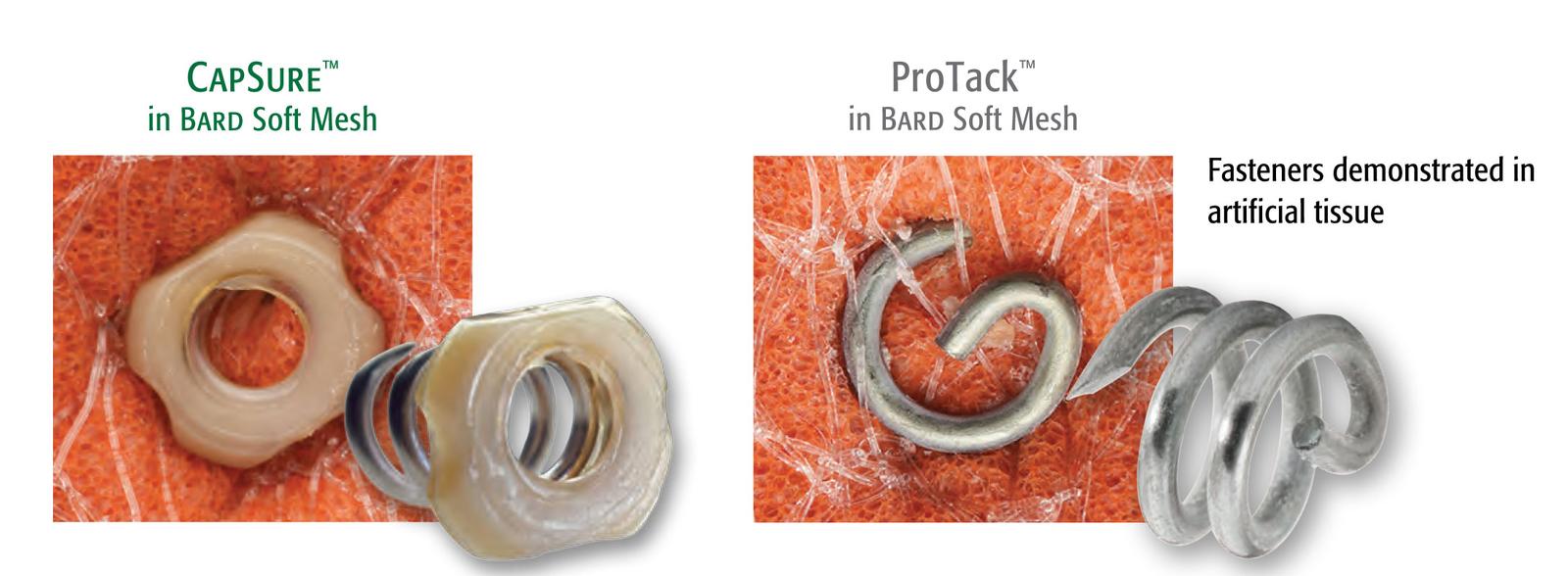
REDEFINED FASTENER DESIGN
316L stainless steel
- 316L stainless steel is a surgical stainless steel commonly used in biomedical implants that are put under pressure including bone screws and prostheses
Smooth PEEK cap
- Cap is made from polyetheretherketone (PEEK). PEEK is an inert organic thermoplastic polymer, considered an advanced biomaterial3
PEEK is used in many medical implants including dental implants, heart valves and stents, and joint prostheses
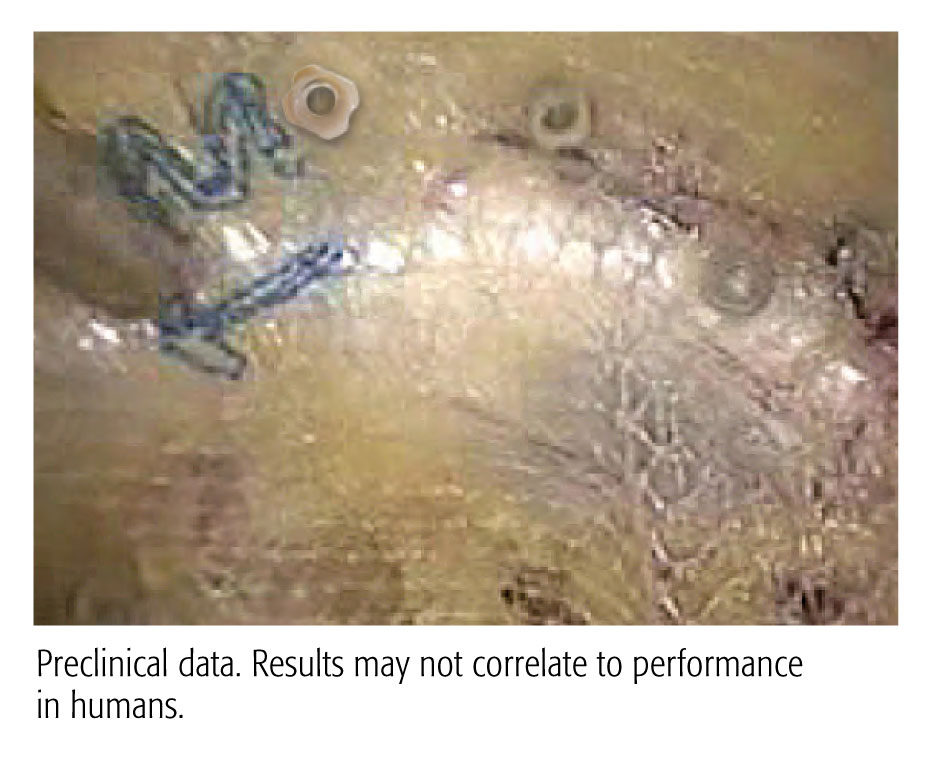
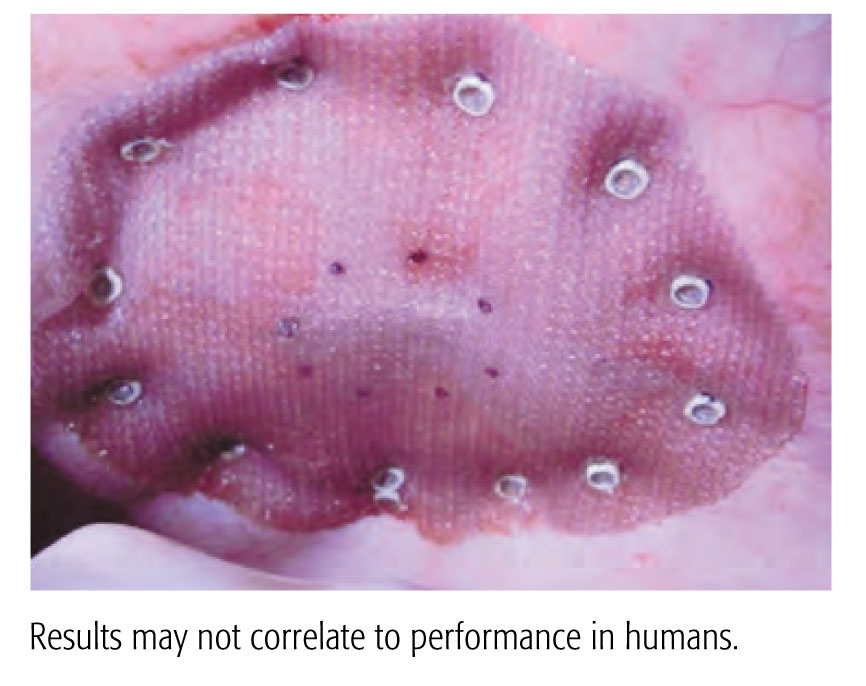
A 90 Day Preclinical Adhesion Study Demonstrated Stronger Results with the CapSure™ Fixation System vs. ProTack™ Fixation System.
Evaluation of a Novel Permanent Capped Helical Coil Fastener in a Porcine Model of Laparoscopic Ventral Hernia Repair
Arnab Majumder, Mojtaba Fayezizadeh, William W. Hope,
Yuri W. Novitsky • Surgical Endoscopy April 2016
- Significantly less adhesion coverage
- Greater percentage of properly engaged fasteners
- Greater mesh/tissue integration
- Shielding exposed fastener points on the visceral mesh surface with polymer caps is suggested by the data to reduce adhesion formation and aid in mesh fixation and integration
Preclinical data. Results may not correlate to performance in humans.
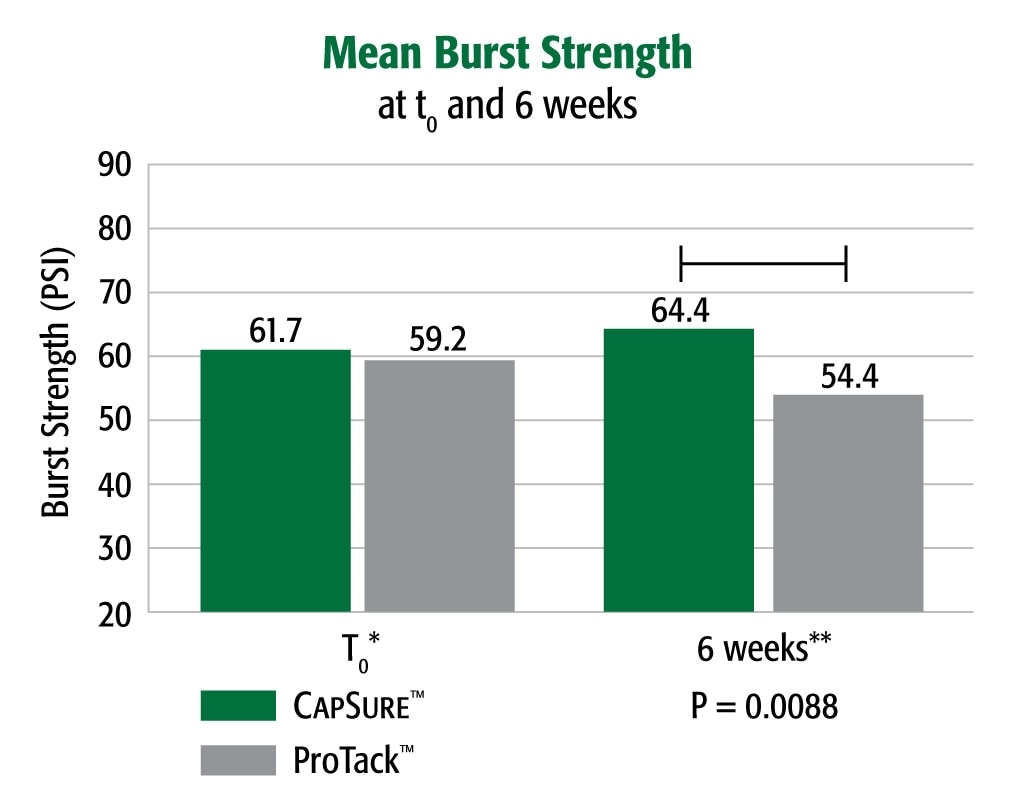
Equivalent Strength to ProTack™ at t0, Greater Strength Over Time
Burst strength testing demonstrated that in a porcine model CapSure™ fixated Ventralight™ ST Mesh had a greater (4.3%) burst strength at t0 and significantly higher (18.4%) peak burst strength at 6 weeks post-implantation than did ProTack™ fixated Ventralight™ ST Mesh at the same time point (p = .0088).
* Porcine abdominal wall tissue:
Animal data may not correlate to performance in humans
* Porcine 6 week implant study:
Animal data may not correlate to performance in humans
Better confidence in your delivery system performance
- Rotary drive system with a comfortable handle and easy to deploy trigger, with an average delivery force that is 30% less than ProTack™1
- Consistent fastener deployment and depth of tissue purchase – preclinical studies demonstrated more favorable fastener seating results versus ProTack™2
- Available in 15 and 30 fastener count devices providing significant savings per device over ProTack™, for repairs where 15 or less fasteners are required
CapSure™ Fasteners have 2X the mesh surface area coverage to hold mesh in place ensuring secure fixation and more visible fasteners. Bench testing with 3DMax™ Light demonstrates that CapSure™ is 15X more likely to retain large pore mesh versus ProTack™1
1. Bench top data. Results many not correlate to performance in humans.
2 Preclinical data. Results may not correlate to performance in humans.
3. Evaluation of a novel permanent capped helical coil fastener in a porcine model of laparoscopic ventral hernia repair. Arnab Majumder, Mojtaba Fayezizadeh, William W. Hope, Yuri W. Novitsky. Surgical Endoscopy. April, 2016.
Indications
The CapSure™ Permanent Fixation System is indicated for the approximation of soft tissue and fixation of surgical mesh to tissues during open or laparoscopic surgical procedures, such as hernia repair.
Contraindications
1. This device is not intended for use except as indicated.
2. Do not use this device where hemostasis cannot be verified visually after application.
3. Contraindications associated with laparoscopic and open surgical procedures relative to mesh fixation apply, including but not limited to: fixation of vascular or neural structures and in ischemic or necrotic tissue.
4. Carefully inspect the area in the vicinity of the tissue being fastened to avoid inadvertent penetration of underlying structures such as bone, nerves, vessels, and viscera. Use of the CapSure™ Permanent Fixation System in the close vicinity of such underlying structures is contraindicated. For reference, the length of the fastener below the fastener head is 3.2 mm, the fastener head is another 1 mm (total 4.2 mm).
5. This device should not be used in tissues that have a direct anatomic relationship to major vascular or nerve structures. This includes the deployment of tacks in the diaphragm in the vicinity of the pericardium, aorta, or inferior vena cava during diaphragmatic hernia repair.
Warnings
1. The CapSure™ Permanent Fixation System is intended for Single Use Only - DO NOT RESTERILIZE. Reuse, reprocessing, resterilization or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the device and may lead to device failure which may result in injury to the patient. Reuse, reprocessing, resterilization or repackaging may also create a risk of contamination of the device and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another. Contamination of the device may lead to injury, illness or death of the patient or end user.
2. Do not use beyond the expiration date on the package.
3. This product is supplied sterile. Inspect the packaging to be sure it is intact and undamaged prior to use.
4. Verify mechanical and electrical compatibility of devices from different manufacturers prior to using them together in a procedure.
5. Prosthetics should be evaluated for compatibility prior to use.
6. Users should be familiar with surgical procedures and techniques involving permanent materials before employing CapSure™ Permanent Fixation System fasteners for wound closure, as the risk of wound dehiscence may vary with the site of application and the material used.
7. As with any implant material, the presence of bacterial contamination may enhance bacterial infectivity. Accepted surgical practice must be followed with respect to drainage and closure of infected or contaminated wounds. After use, the CapSure™ Permanent Fixation System may be a potential biohazard. Handle and dispose of in accordance with any local and federal laws regarding medical waste.
Precautions
1. Please read all instructions before using the CapSure™ Permanent Fixation System.
2. Only persons having adequate medical training and familiarity with surgical techniques should perform surgical procedures. Consult the medical literature relative to technique, complications and hazards prior to any surgical procedure. 3. The CapSure™ Permanent Fixation System can be used with most 5 mm trocars. Ensure compatibility by inserting the device into the trocar prior to introduction into the patient. The CapSure™ Permanent Fixation System should enter and exit the trocar easily without excessive force. The use of too much force could damage the instrument.
4. Adequate counter pressure should be applied on the target area. Avoid placing hand or finger directly over the area where fastener is being deployed to prevent injury.
5. Use caution when applying the CapSure™ fastener over or in proximity to underlying bone, vessels, nerves, or viscera. The intended fixation site should be assessed to ensure that while the tissue is compressed the total distance from the surface of the tissue to any underlying structures is greater than the length of the CapSure™ fastener.
6. Care should be taken not to use excessive counter pressure as this may damage the tissue, the material being fixated, and/or the device.
7. If the device locks and cannot be separated from a fastener that has been deployed into mesh and/or tissue, rotate the device counterclockwise to free the fastener from the tissue and/or to free the device. If the fastener does not deploy properly, remove the device from the patient and test the device in gauze to ensure proper fastener deployment, otherwise discard the device appropriately and use a new CapSure™ Permanent Fixation System. Once proper fastener deployment is confirmed, the device may be reinserted into the patient.
8. The safety and effectiveness of CapSure™ Permanent Fixation System have not been evaluated or established in pregnant or breast-feeding women.
Adverse Reactions.
Adverse reactions and potential complications associated with fixation devices such as the CapSure™ Permanent Fixation System may include, but are not limited to the following: hemorrhage, pain, edema and erythema at wound site; septicemia/infection; hernia recurrence/wound dehiscence, erosion and allergic response in patients with known sensitivities to PEEK and metals contained in 316L stainless steel, including chromium, nickel, copper, and iron.
Please consult package insert for more detailed safety information and instructions for use.
1. Bench top data. Results many not correlate to performance in humans.
2 Preclinical data. Results may not correlate to performance in humans.
3. Evaluation of a novel permanent capped helical coil fastener in a porcine model of laparoscopic ventral hernia repair. Arnab Majumder, Mojtaba Fayezizadeh, William W. Hope, Yuri W. Novitsky. Surgical Endoscopy. April, 2016.
Indications
The CapSure™ Permanent Fixation System is indicated for the approximation of soft tissue and fixation of surgical mesh to tissues during open or laparoscopic surgical procedures, such as hernia repair.
Contraindications
1. This device is not intended for use except as indicated.
2. Do not use this device where hemostasis cannot be verified visually after application.
3. Contraindications associated with laparoscopic and open surgical procedures relative to mesh fixation apply, including but not limited to: fixation of vascular or neural structures and in ischemic or necrotic tissue.
4. Carefully inspect the area in the vicinity of the tissue being fastened to avoid inadvertent penetration of underlying structures such as bone, nerves, vessels, and viscera. Use of the CapSure™ Permanent Fixation System in the close vicinity of such underlying structures is contraindicated. For reference, the length of the fastener below the fastener head is 3.2 mm, the fastener head is another 1 mm (total 4.2 mm).
5. This device should not be used in tissues that have a direct anatomic relationship to major vascular or nerve structures. This includes the deployment of tacks in the diaphragm in the vicinity of the pericardium, aorta, or inferior vena cava during diaphragmatic hernia repair.
Warnings
1. The CapSure™ Permanent Fixation System is intended for Single Use Only - DO NOT RESTERILIZE. Reuse, reprocessing, resterilization or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the device and may lead to device failure which may result in injury to the patient. Reuse, reprocessing, resterilization or repackaging may also create a risk of contamination of the device and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another. Contamination of the device may lead to injury, illness or death of the patient or end user.
2. Do not use beyond the expiration date on the package.
3. This product is supplied sterile. Inspect the packaging to be sure it is intact and undamaged prior to use.
4. Verify mechanical and electrical compatibility of devices from different manufacturers prior to using them together in a procedure.
5. Prosthetics should be evaluated for compatibility prior to use.
6. Users should be familiar with surgical procedures and techniques involving permanent materials before employing CapSure™ Permanent Fixation System fasteners for wound closure, as the risk of wound dehiscence may vary with the site of application and the material used.
7. As with any implant material, the presence of bacterial contamination may enhance bacterial infectivity. Accepted surgical practice must be followed with respect to drainage and closure of infected or contaminated wounds. After use, the CapSure™ Permanent Fixation System may be a potential biohazard. Handle and dispose of in accordance with any local and federal laws regarding medical waste.
Precautions
1. Please read all instructions before using the CapSure™ Permanent Fixation System.
2. Only persons having adequate medical training and familiarity with surgical techniques should perform surgical procedures. Consult the medical literature relative to technique, complications and hazards prior to any surgical procedure. 3. The CapSure™ Permanent Fixation System can be used with most 5 mm trocars. Ensure compatibility by inserting the device into the trocar prior to introduction into the patient. The CapSure™ Permanent Fixation System should enter and exit the trocar easily without excessive force. The use of too much force could damage the instrument.
4. Adequate counter pressure should be applied on the target area. Avoid placing hand or finger directly over the area where fastener is being deployed to prevent injury.
5. Use caution when applying the CapSure™ fastener over or in proximity to underlying bone, vessels, nerves, or viscera. The intended fixation site should be assessed to ensure that while the tissue is compressed the total distance from the surface of the tissue to any underlying structures is greater than the length of the CapSure™ fastener.
6. Care should be taken not to use excessive counter pressure as this may damage the tissue, the material being fixated, and/or the device.
7. If the device locks and cannot be separated from a fastener that has been deployed into mesh and/or tissue, rotate the device counterclockwise to free the fastener from the tissue and/or to free the device. If the fastener does not deploy properly, remove the device from the patient and test the device in gauze to ensure proper fastener deployment, otherwise discard the device appropriately and use a new CapSure™ Permanent Fixation System. Once proper fastener deployment is confirmed, the device may be reinserted into the patient.
8. The safety and effectiveness of CapSure™ Permanent Fixation System have not been evaluated or established in pregnant or breast-feeding women.
Adverse Reactions.
Adverse reactions and potential complications associated with fixation devices such as the CapSure™ Permanent Fixation System may include, but are not limited to the following: hemorrhage, pain, edema and erythema at wound site; septicemia/infection; hernia recurrence/wound dehiscence, erosion and allergic response in patients with known sensitivities to PEEK and metals contained in 316L stainless steel, including chromium, nickel, copper, and iron.
Please consult package insert for more detailed safety information and instructions for use.
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.
We support the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
The BD Learning Academy offers a centralized repository of BD product training, education, and the ability to connect courses to your LMS.
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
1. Bench top data. Results many not correlate to performance in humans.
2 Preclinical data. Results may not correlate to performance in humans.
3. Evaluation of a novel permanent capped helical coil fastener in a porcine model of laparoscopic ventral hernia repair. Arnab Majumder, Mojtaba Fayezizadeh, William W. Hope, Yuri W. Novitsky. Surgical Endoscopy. April, 2016.
Indications
The CapSure™ Permanent Fixation System is indicated for the approximation of soft tissue and fixation of surgical mesh to tissues during open or laparoscopic surgical procedures, such as hernia repair.
Contraindications
1. This device is not intended for use except as indicated.
2. Do not use this device where hemostasis cannot be verified visually after application.
3. Contraindications associated with laparoscopic and open surgical procedures relative to mesh fixation apply, including but not limited to: fixation of vascular or neural structures and in ischemic or necrotic tissue.
4. Carefully inspect the area in the vicinity of the tissue being fastened to avoid inadvertent penetration of underlying structures such as bone, nerves, vessels, and viscera. Use of the CapSure™ Permanent Fixation System in the close vicinity of such underlying structures is contraindicated. For reference, the length of the fastener below the fastener head is 3.2 mm, the fastener head is another 1 mm (total 4.2 mm).
5. This device should not be used in tissues that have a direct anatomic relationship to major vascular or nerve structures. This includes the deployment of tacks in the diaphragm in the vicinity of the pericardium, aorta, or inferior vena cava during diaphragmatic hernia repair.
Warnings
1. The CapSure™ Permanent Fixation System is intended for Single Use Only - DO NOT RESTERILIZE. Reuse, reprocessing, resterilization or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the device and may lead to device failure which may result in injury to the patient. Reuse, reprocessing, resterilization or repackaging may also create a risk of contamination of the device and/or cause patient infection or cross infection, including, but not limited to, the transmission of infectious diseases from one patient to another. Contamination of the device may lead to injury, illness or death of the patient or end user.
2. Do not use beyond the expiration date on the package.
3. This product is supplied sterile. Inspect the packaging to be sure it is intact and undamaged prior to use.
4. Verify mechanical and electrical compatibility of devices from different manufacturers prior to using them together in a procedure.
5. Prosthetics should be evaluated for compatibility prior to use.
6. Users should be familiar with surgical procedures and techniques involving permanent materials before employing CapSure™ Permanent Fixation System fasteners for wound closure, as the risk of wound dehiscence may vary with the site of application and the material used.
7. As with any implant material, the presence of bacterial contamination may enhance bacterial infectivity. Accepted surgical practice must be followed with respect to drainage and closure of infected or contaminated wounds. After use, the CapSure™ Permanent Fixation System may be a potential biohazard. Handle and dispose of in accordance with any local and federal laws regarding medical waste.
Precautions
1. Please read all instructions before using the CapSure™ Permanent Fixation System.
2. Only persons having adequate medical training and familiarity with surgical techniques should perform surgical procedures. Consult the medical literature relative to technique, complications and hazards prior to any surgical procedure. 3. The CapSure™ Permanent Fixation System can be used with most 5 mm trocars. Ensure compatibility by inserting the device into the trocar prior to introduction into the patient. The CapSure™ Permanent Fixation System should enter and exit the trocar easily without excessive force. The use of too much force could damage the instrument.
4. Adequate counter pressure should be applied on the target area. Avoid placing hand or finger directly over the area where fastener is being deployed to prevent injury.
5. Use caution when applying the CapSure™ fastener over or in proximity to underlying bone, vessels, nerves, or viscera. The intended fixation site should be assessed to ensure that while the tissue is compressed the total distance from the surface of the tissue to any underlying structures is greater than the length of the CapSure™ fastener.
6. Care should be taken not to use excessive counter pressure as this may damage the tissue, the material being fixated, and/or the device.
7. If the device locks and cannot be separated from a fastener that has been deployed into mesh and/or tissue, rotate the device counterclockwise to free the fastener from the tissue and/or to free the device. If the fastener does not deploy properly, remove the device from the patient and test the device in gauze to ensure proper fastener deployment, otherwise discard the device appropriately and use a new CapSure™ Permanent Fixation System. Once proper fastener deployment is confirmed, the device may be reinserted into the patient.
8. The safety and effectiveness of CapSure™ Permanent Fixation System have not been evaluated or established in pregnant or breast-feeding women.
Adverse Reactions.
Adverse reactions and potential complications associated with fixation devices such as the CapSure™ Permanent Fixation System may include, but are not limited to the following: hemorrhage, pain, edema and erythema at wound site; septicemia/infection; hernia recurrence/wound dehiscence, erosion and allergic response in patients with known sensitivities to PEEK and metals contained in 316L stainless steel, including chromium, nickel, copper, and iron.
Please consult package insert for more detailed safety information and instructions for use.