The GeoAlign™ Marking System on the Lutonix™ 035 DCB is designed to facilitate repeatable catheter alignment at the lesion and increase procedural efficiency.1
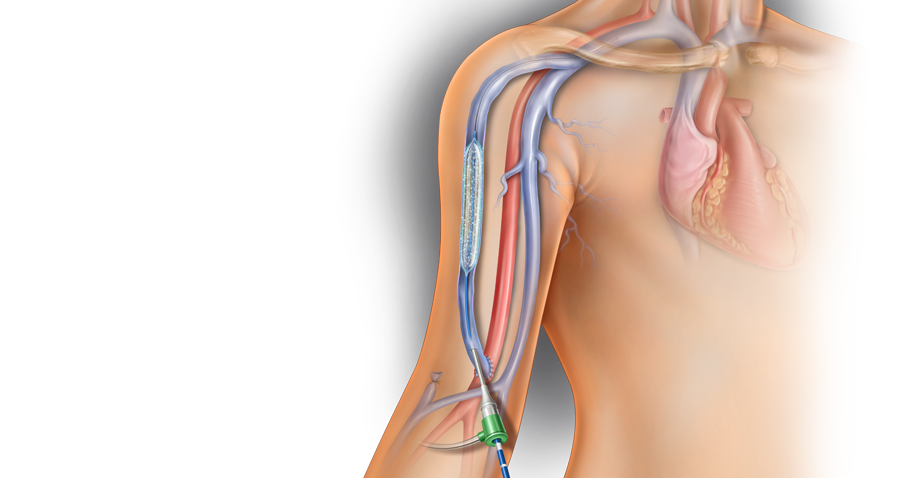

The Lutonix™ 035 Drug Coated Balloon PTA Catheter is indicated for percutaneous transluminal angioplasty (PTA), after pre-dilatation, for treatment of stenotic lesions of dysfunctional native arteriovenous dialysis fistulae that are 4 mm to 12 mm in diameter and up to 80 mm in length.
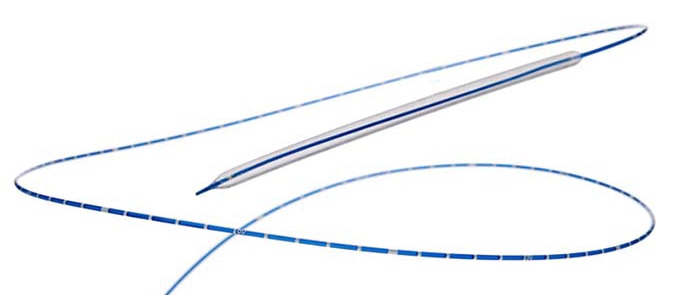
The GeoAlign™ Marking System on the Lutonix™ 035 DCB is designed to facilitate repeatable catheter alignment at the lesion and increase procedural efficiency.1
* Pressures are dependent on the size of the balloon diameters.
1 The GeoAlign™ Marking System is not a replacement for fluoroscopy. The Lutonix™ 035 DCB should always be manipulated under fluoroscopic observation when in the body. Animal study (repeat PTA in swine artery) was performed by 3 physicians who tested the Lutonix™ 035 DCB (no drug) and the Ultraverse™ 035 PTA Catheter, both with GeoAlign™ Markers, to POBA with no GeoAlign™ Markers (n=112, test n=96 (average placement time of 66 seconds), control n=16 (average placement of 90 seconds)). Animal data on file, BD, Tempe AZ. Animal test results may not be indicative of clinical performance. Different test methods may yield different results.
2 Dry Inflate/Shake Bench Test data on file. BD. Tempe, AZ. Shake test measured the average drug content lost after balloon was inflated, and after lightly knocking each device against the sides of the centrifuge tube, left and right, five times. n=5 for both devices tested. Bench test results may not be indicative of actual clinical performance. Different test methods may yield different results.
Please consult product labels and instructions for use for indications, contraindications, hazards, warnings and precautions. ℞ only
BD-90919
The Lutonix AV IDE Trial was the first U.S. trial to evaluate the use of a DCB for the treatment of dysfunctional AV fistulas.
The Lutonix AV Global Registry was a prospective, multi-center, single-arm, real-world registry that assessed the safety and effectiveness of Lutonix™ 035 DCB in a real-world setting.
The Lutonix AV Fistula Cohort included subjects from the Lutonix AV Global Registry who met the Lutonix AV Trial eligibility criteria.
Through the Lutonix™ 035 AV DCB Reintervention-Free Warranty Program, a replacement Lutonix™ 035 AV DCB is offered at no additional charge if a patient is treated with Lutonix™ 035 AV DCB, per the IFU for dysfunctional native AV dialysis fistulae, during the contract period, and returns for a reintervention of the original target lesion within 12 months of the initial treatment.*
1 Lutonix AV IDE Trial. Data on file. BD. Tempe, AZ. At 6 months, treatment with Lutonix™ 035 DCB resulted in a target lesion patency rate of 71.4% versus 63.0% with standard PTA alone. Target lesion primary patency defined as freedom from a clinically driven re-intervention of the target lesion or access thrombosis. The primary effectiveness analysis for superiority of DCB vs. PTA was not met with a one-sided p-value of p = 0.0562. At 30 days, treatment with Lutonix™ 035 DCB was associated with primary safety event rate of 95.0% versus 95.8% with PTA alone. Primary safety defined as freedom from localized or systemic serious adverse events through 30 days that reasonably suggests the involvement of the AV access circuit. The primary safety endpoint for non-inferiority for DCB vs. PTA was met with one-sided p-value of p = 0.0019. Percentages reported are derived from Kaplan-Meier analyses (number at risk for primary safety endpoint was 144, number at risk for primary effectiveness endpoint was 144 at 6 months and 117 at 24 months).
2 Post Hoc Analysis, Lutonix AV Fistula Cohort, a subset of the Lutonix AV Global Registry, which was a prospective, multi-center, single-arm registry that assessed the safety and effectiveness of the Lutonix™ 035 DCB in a real-world setting. N=124. Primary effectiveness endpoint was target lesion primary patency, defined as the interval following treatment until freedom from a clinically driven re-intervention of the target lesion or access thrombosis through 6 months, and is based on Kaplan Meier analysis with 92 subjects at risk. At 30 days, treatment with Lutonix™ 035 DCB was associated with freedom from primary safety event rate of 94.9%, with primary safety defined as freedom from localized or systemic serious adverse events through 30 days that reasonably suggests the involvement of the AV access circuit. Data on file. BD. Tempe, AZ.
3 One limitation of the Lutonix AV Global Registry is that the subjects who met the Lutonix AV IDE eligibility criteria (the AV Fistula Cohort) are primarily White (49%) or Asian (46.9%) and thus are not entirely reflective of a U.S. patient population. Please consult product instructions for use for additional details.
* BD reserves the right to terminate this program at any time. Please note, signed Lutonix™ 035 AV DCB Reintervention-Free Warranty Purchase Agreement terms and conditions apply.
Please consult product labels and instructions for use for indications, contraindications, hazards, warnings and precautions. ℞ only
BD-144648
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.
We support the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
The BD Learning Academy offers a centralized repository of BD product training, education, and the ability to connect courses to your LMS.
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.