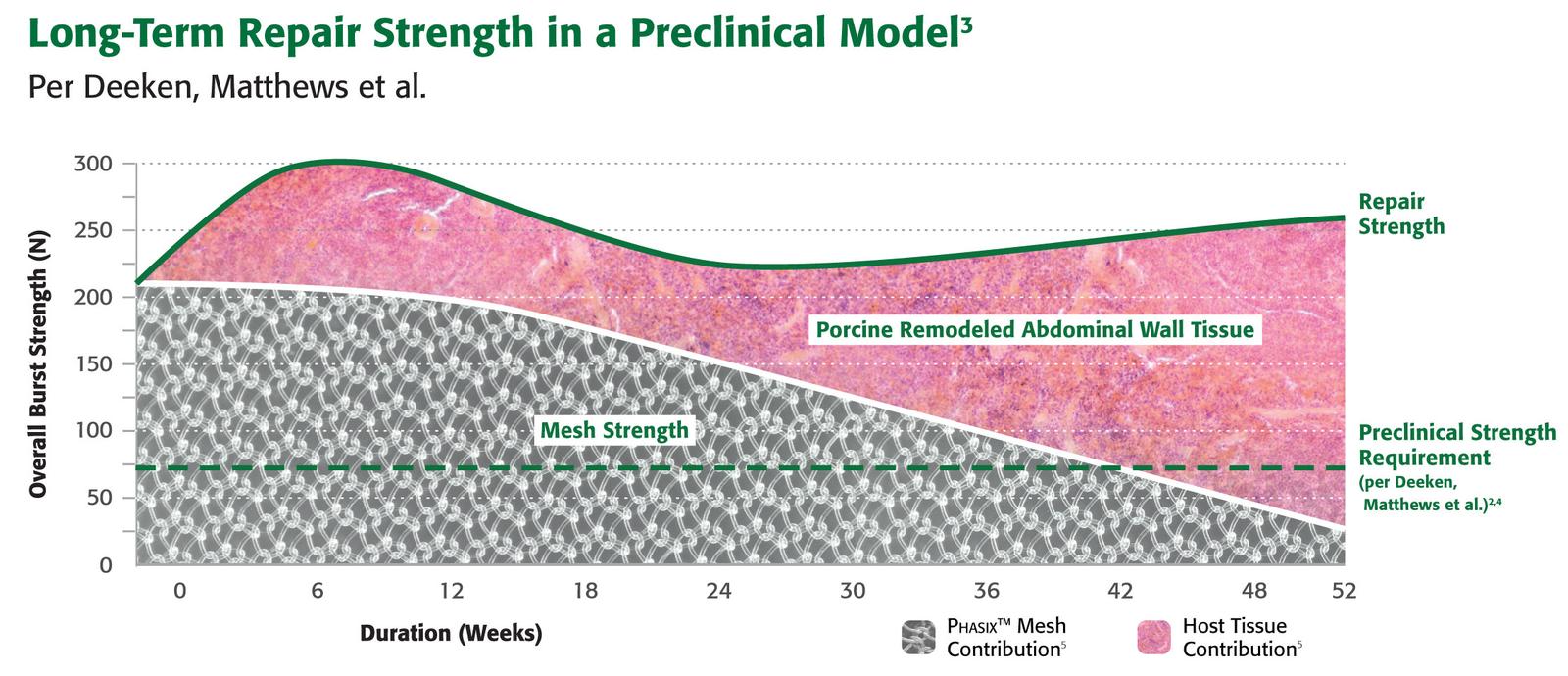
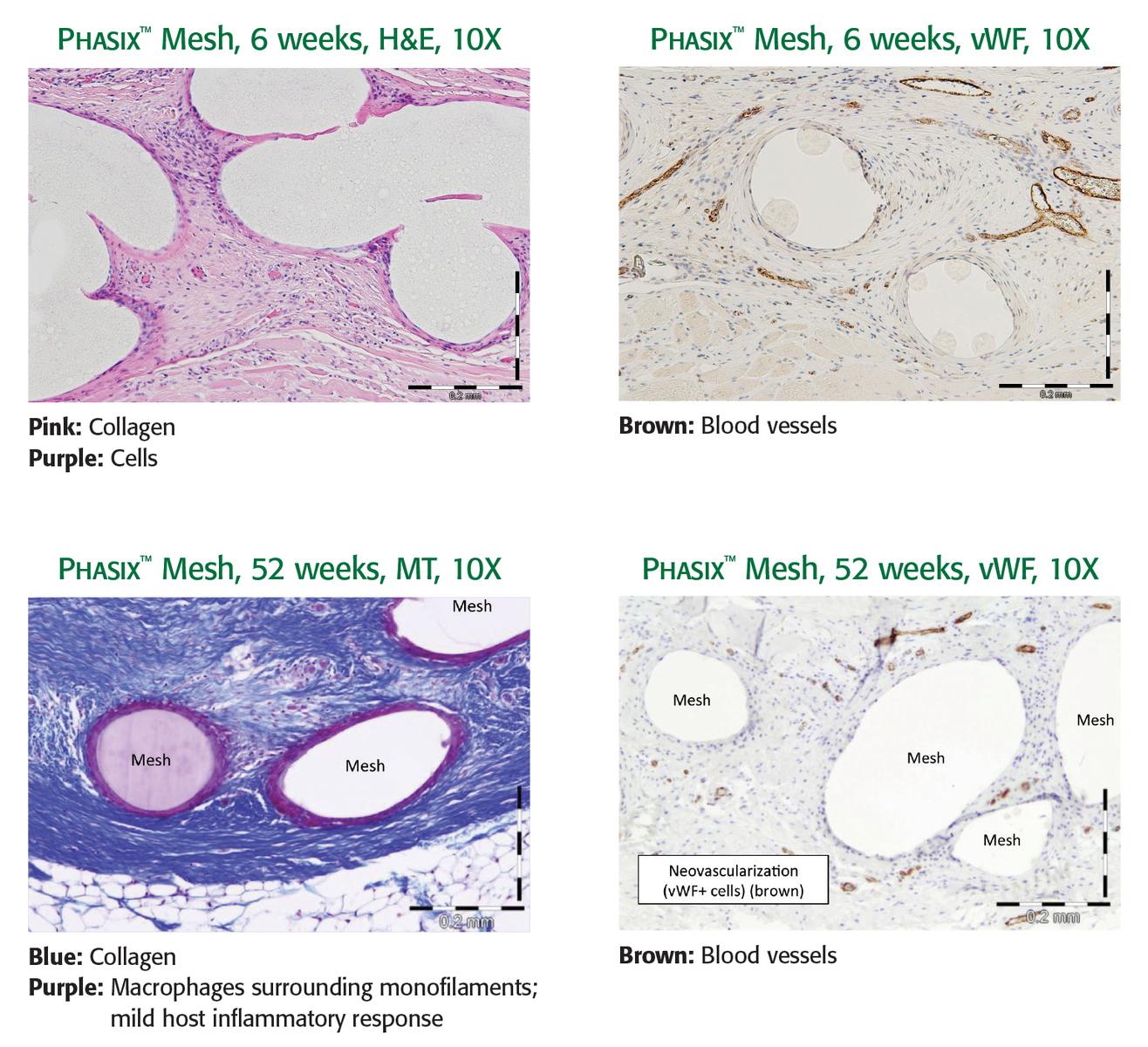
1. Preclinical data on file. Results may not correlate to clinical performance in humans.
2. Deeken CR, Abdo MS, Frisella MM, Matthews BD. “Physicomechanical evaluation of absorbable and nonabsorbable barrier composite meshes for laparoscopic ventral hernia repair.” Surgical Endoscopy 25.5 (2010): 1541-552.
3. Estimated from Standard Curve in (Martin DP, et al. “Characterizationof poly-4-hydroxybutyrate mesh for hernia repair applications.” Journal of Surgical Research. 2013; (184): 766-773
4. Martin, DP. et al. “ Medical applications of poly-4-hydroxybutyrate:a strong flexible absorbable biomaterial. Biochemical Engineering Journal. 2002; December 9
5. Amid PK, Shulman AG, Lichtenstein IL, Hakaha M. Biomaterials for Abdominal Wall Hernia Surgery and Principles of their Applications. Langenbecks Archive Chir. 1994; 379(3): 168-71.
6. Halaweish I, Harth K, Broome AM, Voskerician G, Jacobs MR, Rosen M. Novel In Vitro Model for Assessing Susceptibility of Synthetic Hernia Repair Meshes to Staphylococcus aureus Infection
Using Green Fluorescent Protein-Labeled Bacteria and Modern Imaging Techniques. J Surg Infect (Larchmt). 2010; Oct1(5): 449-54.
Indications
Phasix™ Mesh is indicated to reinforce soft tissue where weakness exists in patients undergoing plastic and reconstructive surgery, or for use in procedures involving soft tissue repair, such as the repair of hernia or other fascial defects that require the addition of a reinforcing or bridging material to obtain the desired surgical result.
Contraindications
Because Phasix™ Mesh is fully resorbable, it should not be used in repairs where permanent wound or organ support from the mesh is required.
Warnings
Phasix™ Mesh must not be put in direct contact with bowel or viscera. The safety and product use for patients with hypersensitivities to tetracycline hydrochloride or kanamycin sulfate is unknown. Use of this device in patients with known allergies to these antibiotics should be avoided. The safety and effectiveness of Phasix™ Mesh in pediatric use has not been evaluated or established. If an infection develops, treat the infection aggressively. An unresolved infection may require removal of the device.
Adverse Reactions
Possible complications include infection, seroma, pain, mesh migration, wound dehiscence, hemorrhage, adhesions, hematoma, inflammation, allergic reaction, extrusion, erosion, fistula formation and recurrence of the hernia or soft tissue defect.
Please consult package insert for more detailed safety information and instructions for use.