References
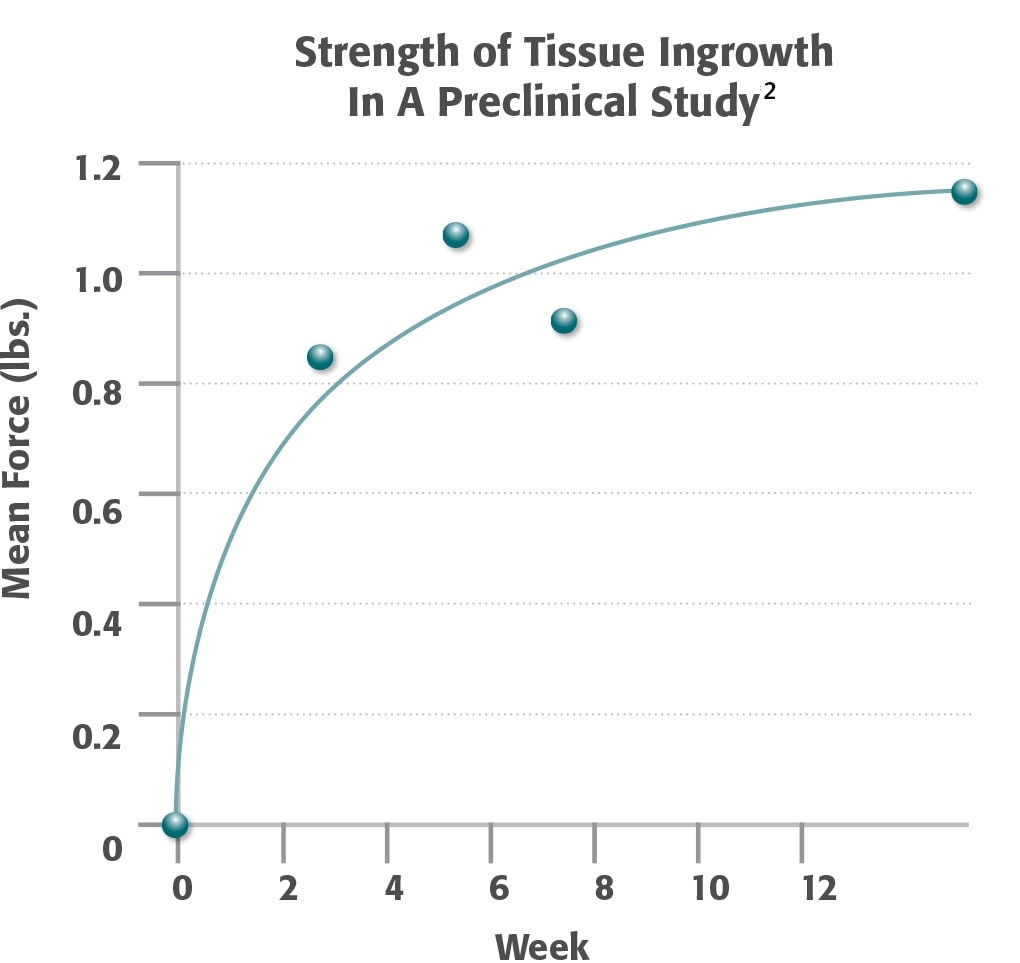
- Data generated in preclinical model. Data may not correlate to performance in humans.
- Majercik, S. et al. “Strength in tissue attachment to mesh after ventral hernia repair with synthetic composite mesh in a porcine model.” Surg Endosc (2006) 20: 1671-1674.
INDICATIONS
The VentrioTM Hernia Patch is indicated for use in the reconstruction of soft tissue deficiencies such as for the repair of hernias.
CONTRADICTIONS
1. Do not use this mesh in infants, children or pregnant women, whereby future growth may be compromised by use of such mesh material.
2. The use of this mesh has not been studied in pregnant or breastfeeding women.
3. Do not use the Ventrio™ Hernia Patch for the reconstruction of cardiovascular defects.
4. Literature reports there may be a possibility for adhesion formation when the polypropylene is placed in contact with the bowel or viscera.
WARNINGS
1. The use of any synthetic mesh or patch in a contaminated or infected wound could lead to fistula formation and/or extrusion of the mesh.
2. If an infection develops, treat the infection aggressively. Consideration should be given regarding the need to remove the mesh. An unresolved infection may require removal of the mesh.
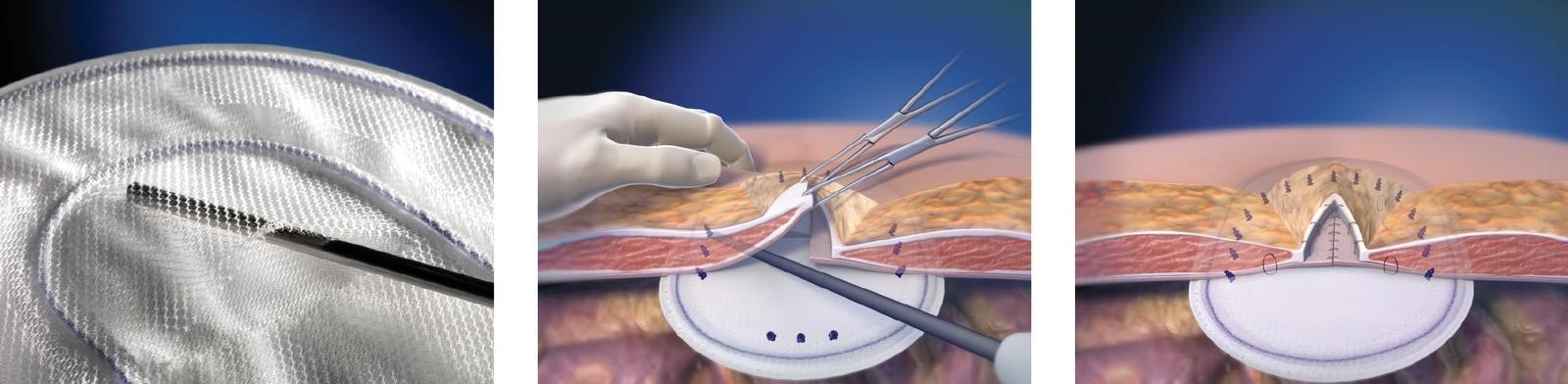
3. Do not cut or reshape the Ventrio™ Hernia Patch, as this could affect its effectiveness. Care should be taken not to cut or nick the SorbaFlex™ PDO monofilament during insertion or fixation. If the SorbaFlex™ PDO monofilament is cut or damaged, the mesh must be removed immediately. Additional complications may include, but are not limited to, bowel or skin perforation and infection.
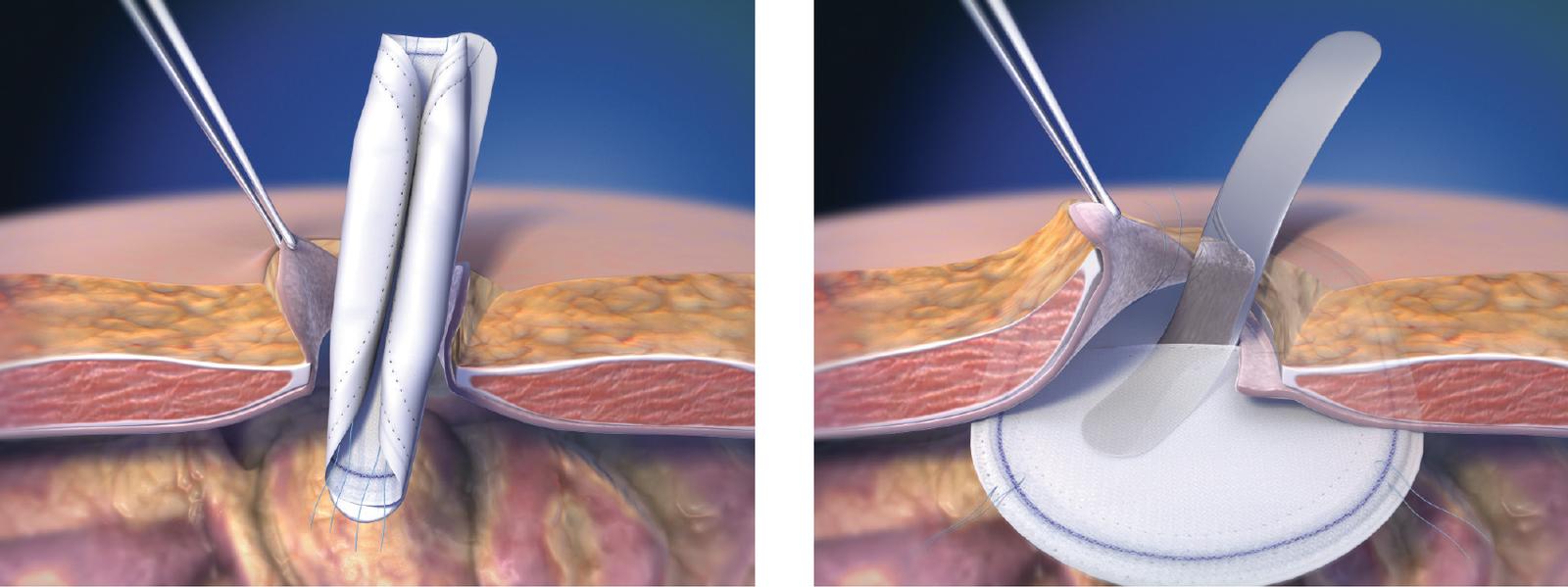
4. Follow proper folding techniques for all patches as described in these Instructions for Use as other folding techniques may break the SorbaFlex™ PDO monofilament.
5. The mesh is supplied sterile. Inspect the packaging to be sure it is intact and undamaged prior to use.
6. This mesh has been designed for single use only. Reuse, resterilization, reprocessing and/or repackaging may compromise the structural integrity and/or essential material and design characteristics that are critical to the overall performance of the device and may lead to device failure which may result in injury to the patient. Reuse, reprocessing, resterilization, or repackaging may also create a risk of contamination of the device and/or cause patient infection or cross infection, including, but not limitedto, the transmission of infectious diseases from one patient to another. Contamination of the device may lead to injury, illness, or death of the patient or end user.
7. Product should be used once exterior foil pouch has been opened. Do not store for later use. Unused portions of mesh should be discarded.
8. Ensure proper orientation; the solid white surface (ePTFE) must be oriented against the bowel or sensitive organs. Do not place the mesh surface against the bowel. There may be a possibility for adhesion formation when mesh is placed in direct contact with the bowel or viscera.
9. Careful attention to the Ventrio™ Hernia Patch handling, fixation, and suture technique is most important in the presence of known or suspected wound contamination or infection.
10. To prevent recurrences when repairing hernias, the mesh should be sized with appropriate overlap for the size and location of the defect, taking into consideration any additional clinical factors applicable to the patient. Careful attention to mesh fixation placement and spacing will help prevent excessive tension or gap formation between the mesh and fascial tissue.
11. To ensure a strong repair, the mesh should be secured through the polypropylene mesh structure. Suturing or tacking on sealed edge of mesh alone is not recommended.
12. This mesh is not for the use of pelvic organ prolapse via transvaginal approach.
13. This mesh is not for the use of treatment of stress urinary incontinence.
PRECAUTIONS
1. Please read all instructions prior to use.
2. Only physicians qualified in the appropriate surgical techniques should use this prosthesis.
3. Care should be taken not to cut or nick the SorbaFlex™ PDO monofilament during fixation.
4. Davol™ fixation devices and/or non- absorbable monofilament sutures are recommended to properly secure the prosthesis. If other fixation devices are used, they must be indicated for use in hernia repair. Care should be taken to ensure that the patch is adequately fixated to the abdominal wall. If necessary, additional fasteners and/or sutures should be used.
ADVERSE REACTIONS
Possible complications include, but are not limited to, seroma, adhesions, hematomas, pain, infection, inflammation, extrusion, erosion, migration, fistula formation, and recurrence of the hernia or soft tissue defect. If the SorbaFlex™ PDO monofilament is cut or damaged during insertion or fixation, additional complications may include bowel or skin perforation and infection.