-
SureStep™ System, Lubri-Sil™ Foley Catheter Tray (Silicone, 18F)
Closed system tray with Urine Bag, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a947318
-
SureStep™ System, Lubri-Sil™ Foley Catheter Tray (Silicone, 16F)
Closed system tray with Urine Bag, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a947316
-
SureStep™ System, Lubri-Sil™ Foley Catheter Tray (Silicone, 14F)
Closed system tray with Urine Bag, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a947314
-
SureStep™ System, Lubri-Sil™ Foley Catheter Tray (Silicone, 18F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a942218
-
SureStep™ System, Lubri-Sil™ Foley Catheter Tray (Silicone, 16F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a942216
-
SureStep™ System, Lubri-Sil™ Foley Catheter Tray (Silicone, 14F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a942214
-
SureStep™ System, Lubri-Sil™ Foley Catheter Tray (Silicone, 12F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a942212
-
SureStep™ System, Lubri-Sil™ Foley Catheter Tray (Silicone, 10F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating and 3cc Balloon
SKU/REF a942210
-
SureStep™ System, Lubri-Sil™ Foley Catheter Tray (Silicone, 8F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating and 3cc Balloon
SKU/REF a942208
-
SureStep™ System, Lubri-Sil™ Foley Catheter Tray (Silicone, 6F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating and 1.5cc Balloon
SKU/REF a942206
-
SureStep™ System, Lubri-Sil™ Foley Catheter Tray (Silicone, 16F)
Closed system tray with Urine Meter and Temperature-Sensing (Molex Connector) Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a909216m
-
SureStep™ System, Lubri-Sil™ Foley Catheter Tray (Silicone, 14F)
Closed system tray with Urine Meter and Temperature-Sensing (Molex Connector) Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a909214m
-
SureStep™ System, Add-a-Foley Complete Care™ Tray (no catheter included)
Add-a-Foley Tray with Urine Meter, StatLock™ Stabilization Device
SKU/REF a904400a
-
SureStep™ System, Bardex™ Lubricath Foley Catheter Tray (Latex, 18F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a902918
-
SureStep™ System, Bardex™ Lubricath Foley Catheter Tray (Latex, 16F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a902916
-
SureStep™ System, Bardex™ Lubricath Foley Catheter Tray (Latex, 14F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a902914
-
SureStep™ System, Uncoated Silicone Foley Catheter Tray (Silicone, 18F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with 5cc Balloon
SKU/REF a902418
-
SureStep™ System, Uncoated Silicone Foley Catheter Tray (Silicone, 16F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with 5cc Balloon
SKU/REF a902416
-
SureStep™ System, Uncoated Silicone Foley Catheter Tray (Silicone, 14F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with 5cc Balloon
SKU/REF a902414
-
SureStep™ System, Bardex™ Lubricath Foley Catheter Tray (Latex, 18F)
Closed system tray with Urine Bag, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a899918
-
SureStep™ System, Bardex™ Lubricath Foley Catheter Tray (Latex, 16F)
Closed system tray with Urine Bag, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a899916
-
SureStep™ System, Bardex™ Lubricath Foley Catheter Tray (Latex, 14F)
Closed system tray with Urine Bag, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a899914
-
SureStep™ System, Uncoated Silicone Foley Catheter Tray (Silicone, 18F)
Closed system tray with Urine Bag, StatLock™ Stabilization Device, and Foley Catheter with 5cc Balloon
SKU/REF a897518
-
SureStep™ System, Uncoated Silicone Foley Catheter Tray (Silicone, 16F)
Closed system tray with Urine Bag, StatLock™ Stabilization Device, and Foley Catheter with 5cc Balloon
SKU/REF a897516
-
SureStep™ System, Uncoated Silicone Foley Catheter Tray (Silicone, 14F)
Closed system tray with Urine Bag, StatLock™ Stabilization Device, and Foley Catheter with 5cc Balloon
SKU/REF a897514
-
SureStep™ System, Add-a-Foley Complete Care™ Tray (no catheter included)
Add-a-Foley Tray with Urine Bag, StatLock™ Stabilization Device
SKU/REF a399400a
-
SureStep™ System, Bardex™ Lubricath Foley Catheter Tray (Latex, 16F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Temperature-Sensing (Monoplug Connector) Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a344916
-
SureStep™ System, Lubri-Sil™ I.C. Foley Catheter Tray (Silicone, 18F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Temperature-Sensing (Molex Connector) Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a319518am
-
SureStep™ System, Lubri-Sil™ I.C. Foley Catheter Tray (Silicone, 16F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Temperature-Sensing (Molex Connector) Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a319516am
-
SureStep™ System, Lubri-Sil™ I.C. Foley Catheter Tray (Silicone, 14F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Temperature-Sensing (Molex Connector) Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a319514am
-
SureStep™ System, Bardex™ I.C. Foley Catheter Tray (Latex, 18F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Temperature-Sensing (Molex Connector) Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a319418am
-
SureStep™ System, Bardex™ I.C. Foley Catheter Tray (Latex, 16F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Temperature-Sensing (Molex Connector) Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a319416am
-
SureStep™ System, Bardex™ I.C. Foley Catheter Tray (Latex, 14F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Temperature-Sensing (Molex Connector) Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a319414am
-
SureStep™ System, Lubri-Sil™ I.C. Complete Care™ Foley Catheter Tray (Silicone, 18F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Coude Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a307418a
-
SureStep™ System, Lubri-Sil™ I.C. Complete Care™ Foley Catheter Tray (Silicone, 16F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Coude Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a307416a
-
SureStep™ System, Bardex™ Lubricath Foley Catheter Tray (Latex, 18F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Coude Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a304918
-
SureStep™ System, Bardex™ Lubricath Foley Catheter Tray (Latex, 16F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Coude Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a304916
-
SureStep™ System, Bardex™ Lubricath Foley Catheter Tray (Latex, 14F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Coude Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a304914
-
SureStep™ System, Bardex™ I.C. Complete Care™ Foley Catheter Tray (Latex, 16F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a304716a
-
SureStep™ System, Bardex™ I.C. Complete Care™ Foley Catheter Tray (Latex, 14F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a304714a
-
SureStep™ System, Bardex™ I.C. Complete Care™ Foley Catheter Tray (Latex, 16F)
Closed system tray with Urine Bag, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a304416a
-
SureStep™ System, Add-a-Foley Complete Care™ Tray (no catheter included)
Add-a-Foley Tray with Urine Meter, StatLock™ Stabilization Device
SKU/REF a304400a
-
SureStep™ System, Lubri-Sil™ I.C. Complete Care™ Foley Catheter Tray (Silicone, 18F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a303418a
-
SureStep™ System, Lubri-Sil™ I.C. Complete Care™ Foley Catheter Tray (Silicone, 16F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a303416a
-
SureStep™ System, Lubri-Sil™ I.C. Complete Care™ Foley Catheter Tray (Silicone, 14F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a303414a
-
SureStep™ System, Bardex™ I.C. Complete Care™ Foley Catheter Tray (Latex, 18F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a303318a
-
SureStep™ System, Bardex™ I.C. Complete Care™ Foley Catheter Tray (Latex, 16F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a303316a
-
SureStep™ System, Bardex™ I.C. Complete Care™ Foley Catheter Tray (Latex, 14F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a303314a
-
SureStep™ System, Lubri-Sil™ I.C. Complete Care™ Foley Catheter Tray (Silicone, 18F)
Closed system tray with Urine Bag, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a300418a
-
SureStep™ System, Lubri-Sil™ I.C. Complete Care™ Foley Catheter Tray (Silicone, 16F)
Closed system tray with Urine Bag, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a300416a
-
SureStep™ System, Lubri-Sil™ I.C. Complete Care™ Foley Catheter Tray (Silicone, 14F)
Closed system tray with Urine Bag, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a300414a
-
SureStep™ System, Bardex™ I.C. Complete Care™ Foley Catheter Tray (Latex, 18F)
Closed system tray with Urine Bag, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a300318a
-
SureStep™ System, Bardex™ I.C. Complete Care™ Foley Catheter Tray (Latex, 16F)
Closed system tray with Urine Bag, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a300316a
-
SureStep™ System, Bardex™ I.C. Complete Care™ Foley Catheter Tray (Latex, 14F)
Closed system tray with Urine Bag, StatLock™ Stabilization Device, and Foley Catheter with Bard® Hydrogel Coating & Bactiguard® Coating and 5cc Balloon
SKU/REF a300314a
-
SureStep™ System, Bardex™ Lubricath Foley Catheter Tray (Latex, 18F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Temperature-Sensing (Molex Connector) Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a119418m
-
SureStep™ System, Bardex™ Lubricath Foley Catheter Tray (Latex, 16F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Temperature-Sensing (Molex Connector) Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a119416m
-
SureStep™ System, Lubri-Sil™ Foley Catheter Tray (Silicone, 18F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Temperature-Sensing (Molex Connector) Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a119218m
-
SureStep™ System, Lubri-Sil™ Foley Catheter Tray (Silicone, 16F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Temperature-Sensing (Molex Connector) Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a119216m
-
SureStep™ System, Lubri-Sil™ Foley Catheter Tray (Silicone, 14F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Temperature-Sensing (Molex Connector) Foley Catheter with Bard® Hydrogel Coating and 5cc Balloon
SKU/REF a119214m
-
SureStep™ System, Uncoated Silicone Foley Catheter Tray (Silicone, 10F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Temperature-Sensing (Monoplug Connector) Foley Catheter with 3cc Balloon
SKU/REF a119110
-
SureStep™ System, Uncoated Silicone Foley Catheter Tray (Silicone, 8F)
Closed system tray with Urine Meter, StatLock™ Stabilization Device, and Temperature-Sensing (Monoplug Connector) Foley Catheter with 3cc Balloon
SKU/REF a119108
For sales inquiries, technical support and customer support complete the following form:

- Overview
- Step-by-Step Process
- Training & Education
- Products & Accessories
- Resources
Following the directions is as easy as A, B, C
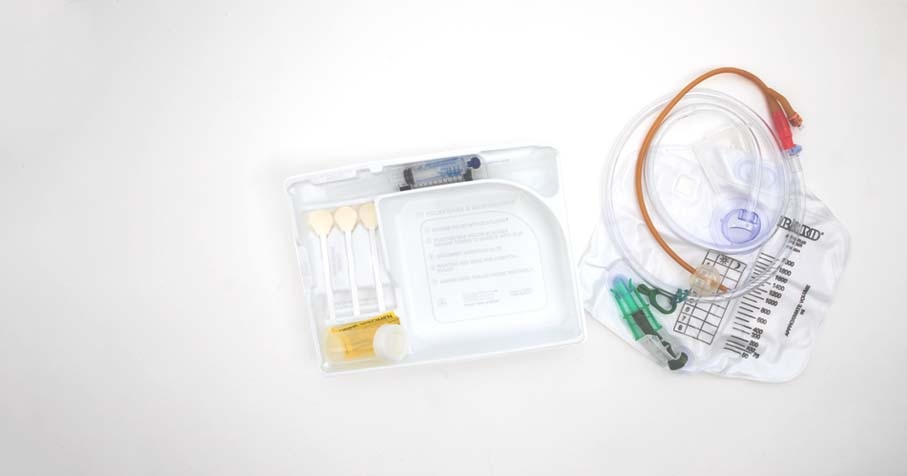
The SureStep™ Foley Tray System was designed to help provide a consistent step-by-step process to guide the clinician through the Foley insertion process. The recommended steps are displayed directly on the tray, starting with patient preparation all the way to catheter insertion and maintenance.
Remember the ABC’s of catheter insertion:
Step A: STOP! Cleanse the peri-urethral area first
- A clearly labeled reminder to perform peri-urethral cleansing.
- Comprehensive directions in the form of four simple steps to perform peri-care.
- Complete isolation of peri-prep components from the insertion components.
STEP B: Utilize proper aseptic technique
- Open CSR wrap
- Don sterile gloves
- Place underpad
- Place fenestrated drape
- Follow tray instructions
SureStep™ Foley Catheter Tray instructions

STEP C: Foley Care & Maintenance
- Secure Foley with StatLock™ Stabilization Device
- Position bag below bladder, secure tubing to sheets with clip
- Document insertion date
- Maintain red seal per hospital policy
- Assess need for catheter routinely
Make BD and the SureStep™ Foley Tray System part of your CAUTI prevention plan!
Fill out the form below to get started.
- United States of America. Department of Health and Human Services. Centers for Disease Control and Prevention. Guideline for Prevention of Catheter-Associated Urinary Tract Infections 2009. By Carolyn V. Gould, Craig A. Umscheid, et al. Healthcare Infection Control Practices Advisory Committee, 2009.
Specifications subject to change without notice.
Foley catheters are intended for use in the drainage and/or collection and/or measurement of urine.
Cautions: Latex Foley catheters contain natural rubber latex which may cause allergic reactions.
Federal (U.S.A.) law restricts these devices to sale by or on the order of a physician.
Warnings: On catheter, do not use ointments or lubricants having a petrolatum base. They will damage the catheter and may cause balloon to burst.
After use, this product may be a potential biohazard. Handle and dispose of in accordance with accepted medical practices and applicable local, state and federal laws and regulations.
Visually inspect the product for any imperfections or surface deterioration prior to use. If package is opened or if any imperfection or surface deterioration is observed, do not use.
Please consult product label and insert for any indications, contraindications, hazards, warnings, cautions and directions for use.
Following the directions is as easy as A, B, C
The SureStep™ Foley Tray System was designed to help provide a consistent step-by-step process to guide the clinician through the Foley insertion process. The recommended steps are displayed directly on the tray, starting with patient preparation all the way to catheter insertion and maintenance.
Remember the ABC’s of catheter insertion:
Includes Peri-Care Kit:
The Peri-Care Kit contains:
- A clearly labeled reminder to perform peri-urethral cleansing.
- Comprehensive directions in the form of four simple steps to perform peri-care.
- Complete isolation of peri-prep components from the insertion components.
Facilitates Aseptic Technique:
The SureStep™ Foley Tray System was designed to facilitate aseptic technique due to:
- A tray design that makes it easy to access all of the components.
- Foam swabs designed to offer greater coverage than the pre-saturated swabsticks in the Bard™ Advance Tray Platform.
- On tray instructions designed to guide the user through aseptic insertion techniques.
Aligns with CDC Recommendations:
The SureStep™ Foley Tray System aligns with the following CDC Category IB recommendations1:
- Secure – Properly secure indwelling catheters after insertion to prevent movement and urethral traction.
- Closed System – Maintain a closed drainage system.
- Bag Management – Keep the collection bag below the bladder at all times.
- United States of America. Department of Health and Human Services. Centers for Disease Control and Prevention. Guideline for Prevention of Catheter-Associated Urinary Tract Infections 2009. By Carolyn V. Gould, Craig A. Umscheid, et al. Healthcare Infection Control Practices Advisory Committee, 2009.
Specifications subject to change without notice.
Foley catheters are intended for use in the drainage and/or collection and/or measurement of urine.
Cautions: Latex Foley catheters contain natural rubber latex which may cause allergic reactions.
Federal (U.S.A.) law restricts these devices to sale by or on the order of a physician.
Warnings: On catheter, do not use ointments or lubricants having a petrolatum base. They will damage the catheter and may cause balloon to burst.
After use, this product may be a potential biohazard. Handle and dispose of in accordance with accepted medical practices and applicable local, state and federal laws and regulations.
Visually inspect the product for any imperfections or surface deterioration prior to use. If package is opened or if any imperfection or surface deterioration is observed, do not use.
Please consult product label and insert for any indications, contraindications, hazards, warnings, cautions and directions for use.
BD’s Clinical Team would like to work with your hospital to deliver product training and education. BD’s Clinical Connect Training and Education Program can help your hospital achieve and sustain the right results.
Participate in a program, at the time and place, that works best for you and your team.
- Access urological management product training and education on the go.
- Comprehensive product training assessments available for BD Urology to promote correct product use.
- Experience technologically innovative urological management product training and education.
Specifications subject to change without notice.
For the latest information, always check the “Instructions for Use” that comes packaged with the product. If you are a consumer seeking more information, please consult your healthcare provider.
Specifications subject to change without notice.
For the latest information, always check the “Instructions for Use” that comes packaged with the product. If you are a consumer seeking more information, please consult your healthcare provider.
Our collection of literature on industries and on our offerings gives you information you can use to continue striving for excellence.
We support the healthcare industry with market-leading products and services that aim to improve care while lowering costs. We host and take part in events that excel in advancing the world of health™.
The BD Learning Academy offers a centralized repository of BD product training, education, and the ability to connect courses to your LMS.
We promote clinical excellence by providing various resources on best practices, clinical innovations and industry trends in healthcare.
Specifications subject to change without notice.
For the latest information, always check the “Instructions for Use” that comes packaged with the product. If you are a consumer seeking more information, please consult your healthcare provider.
