*as of September 2025
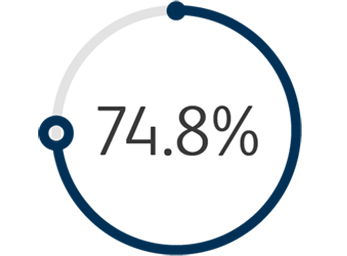
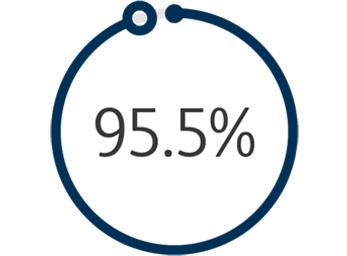
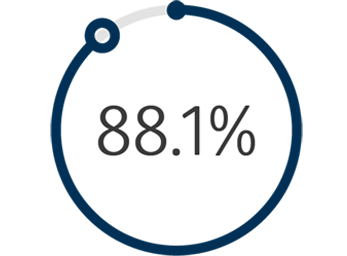
1 The Venovo™ Venous Stent System was studied in the global VERNACULAR clinical trial, which was a prospective, multi-center, non-randomized, single-arm study of 170 patients. The primary effectiveness endpoint of the study was primary patency (PP) at 12 months post-index procedure, defined as: freedom from target vessel revascularization and freedom from thrombus occlusion and stenosis > 50% as measured by diagnostic ultrasound. Patients who received a Venovo™ Venous Stent had a weighted 12-month PP rate of 88.6%, demonstrating a statistically significant difference from a literature-derived performance goal (PG) of 74%. The primary safety endpoint was freedom from major adverse events (MAE) through 30 days post-index procedure. Freedom from MAE through 30 days was 93.5%, demonstrating a statistically significant difference from a literature-derived PG of 89%. There were no stent migrations associated with CEC-adjudicated events at the 30-day primary safety endpoint or through 36 months. Dake MD, O’Sullivan G, Shammas NW, et al. Three-year results from the Venovo venous stent study for the treatment of iliac and femoral vein obstruction. Cardiovasc Intervent Radiol. 2021;44(12):1918-1929. doi: 10.1007/s00270-021-02975-2
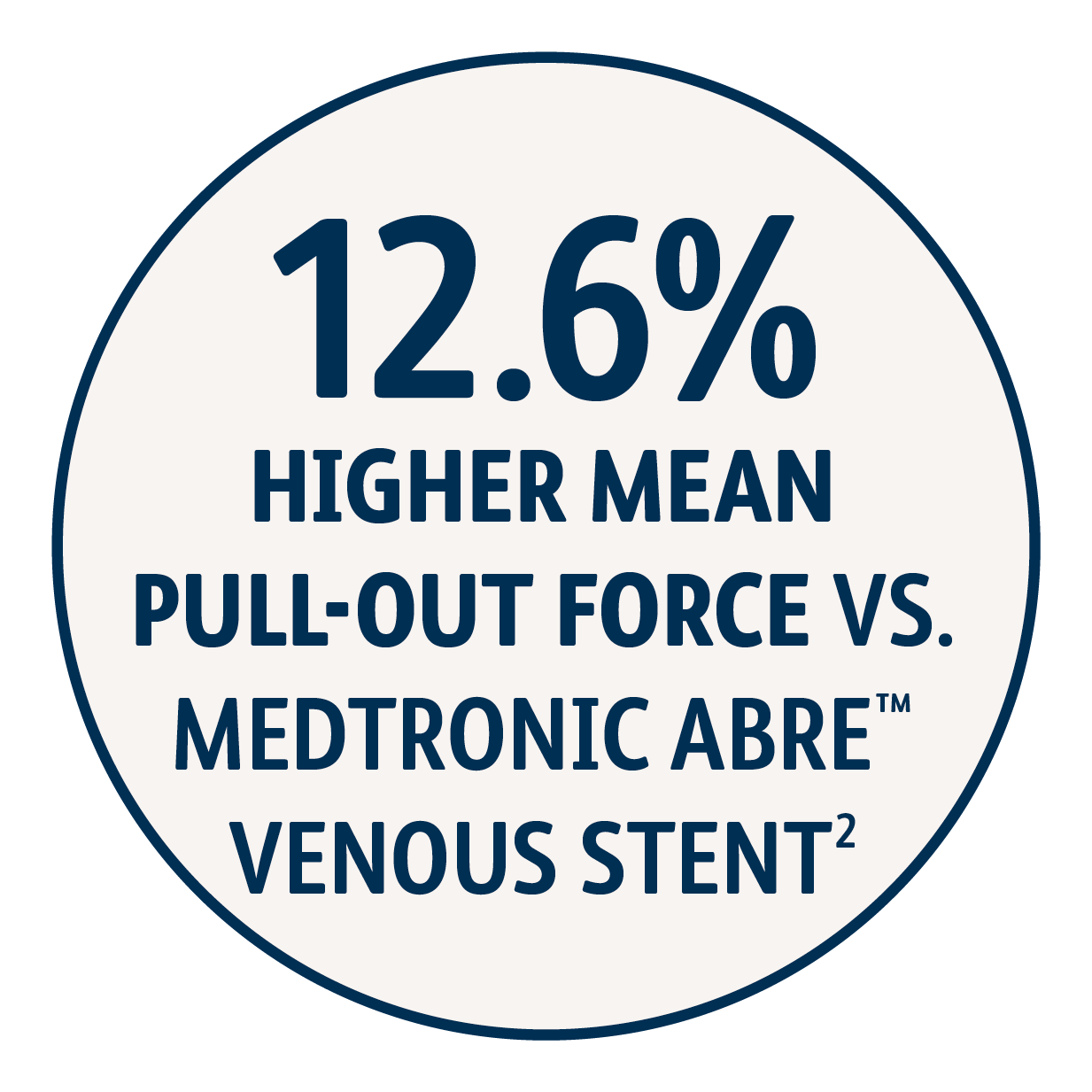
2 Simulated use data on file. BD, Tempe, AZ. Results may not be predictive of actual clinical outcomes. Different test methods may yield different results. Venovo™ Stent (14 mm x 100 mm, N=6), Medtronic Abre™ Stent (14 mm x 100 mm, N=6). The maximum pull-out force of an implant from a silicone tubing section at 1 mm oversizing and at an overlap length of 40 mm was measured. A higher pull-out force is interpreted as higher migration resistance. Venovo™ Stent demonstrated higher mean pull-out force (0.107 N/mm) compared to the Medtronic Abre™ Stent (0.095 N/mm).
3 Simulated use data on file. BD, Tempe, AZ. Results may not be predictive of actual clinical outcomes. Different test methods may yield different results. Venovo™ Stent (14 mm x 100 mm, N=6), Cook Zilver® Vena™ Stent (14 mm x 100 mm, N=6). The pull-out force of an implant from a silicone tubing section at the recommended minimum oversizing and at a defined overlap length was measured. A higher pull-out force is interpreted as higher migration resistance. Venovo™ Stent demonstrated higher mean pull-out force (0.110 N/mm) compared to the Cook Zilver® Vena™ Stent (0.038 N/mm).
BD - 23533v4