BD® Peripheral IV Catheter Devices
One peripheral IV catheter portfolio.
Every patient. Every need.

- Overview
- PIVC Excellence
The world’s leading peripheral IV
catheter portfolio. Because every
patient matters.
Each patient has unique vascular access needs, and every clinician deserves confidence in their tools.
The BD® Peripheral IV Catheter (PIVC) portfolio offers a comprehensive range of clinically-differentiated solutions—from standard safety catheters to advanced closed IV systems and beyond—backed by comprehensive education, expert-led training and support to help optimize patient care and clinical practices across the continuum.
Because every patient deserves reliable vascular access from the start.
The global leader in PIVC solutions
More hospitals trust BD for vascular access than any other company
Designed for every need
BD offers a broad range of PIVC products to meet a variety of clinical needs and preferences
Support for each step
Comprehensive education, expert-led training and support from selection to implementation and beyond
Legacy of innovation firsts
From the first safety-engineered IV catheter to today’s advanced closed systems, BD continues to set industry standards
To learn more about the benefits of using a closed system peripheral IV, click below to watch our webinar.
Comprehensive portfolio of clinically-differentiated peripheral IV catheters
-
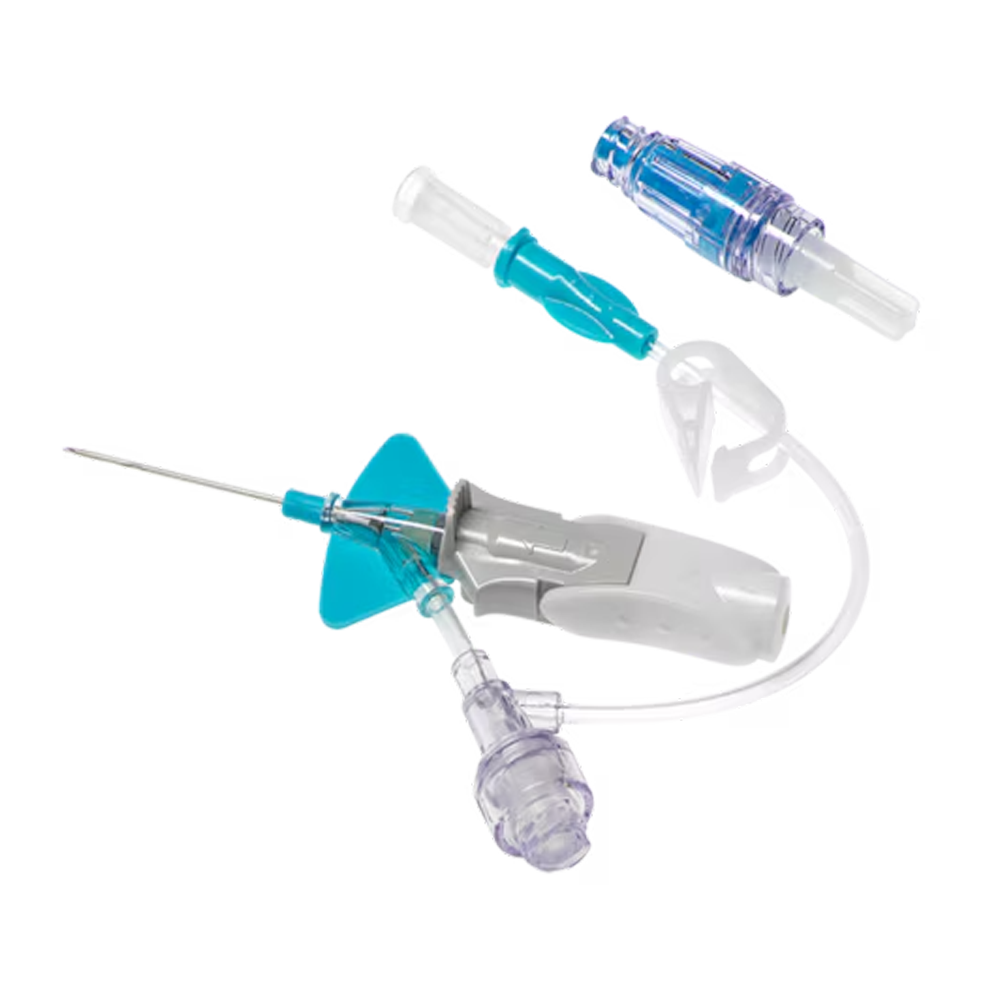
Specifically designed for needle-free blood collection using the compatible PIVO™ Pro Needle-Free Blood Collection Device
-
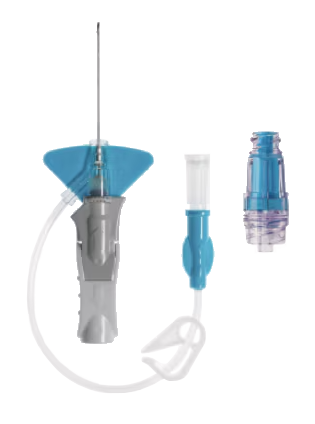
Clinically demonstrated longer dwell times and reduced overall complication rates. With an integrated tubing and stabilization platform, it is designed to reduce manipulation and movement at the site and has been shown to reduce dislodgement and phlebitis*
-
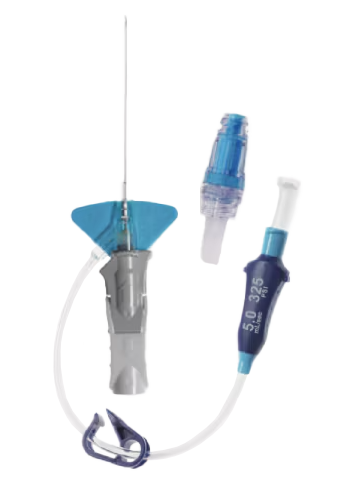
The only PIVC with a diffusion catheter tip specifically designed for power injection and to enable contrast-enhanced CT capability with smaller IV catheters without increasing the risk of complications**
-

Designed to stop the flow of blood from the catheter hub at insertion,† reducing the potential risk of infection and contamination related to blood exposure and removes the need for venous compression†
-

The Insyte Autoguard™ Shielded IV Catheter is designed to provide safety activation with a push of a button and is completely encapsulated after activation to reduce the risk of needlestick injury.
-
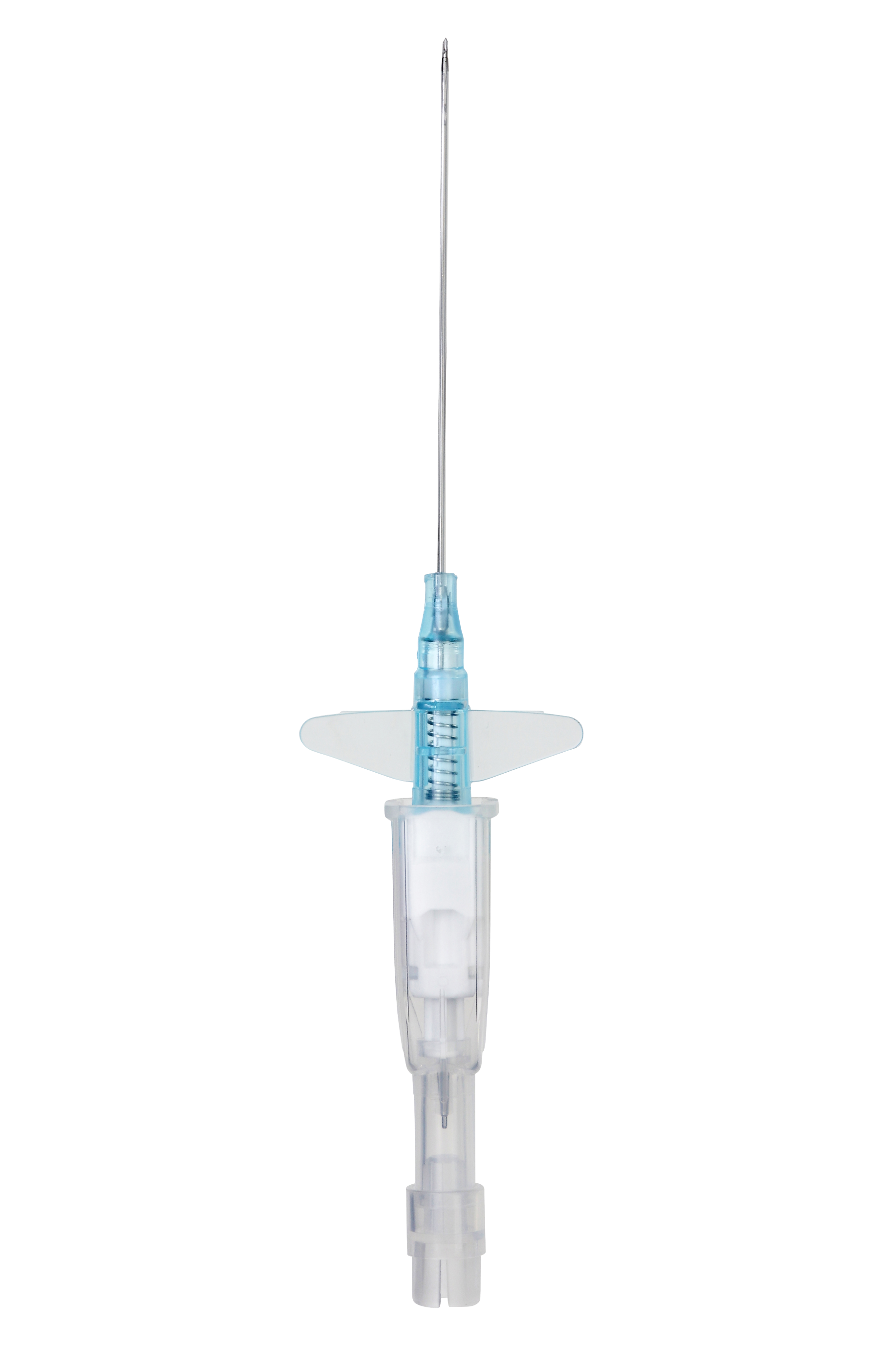
Automatically shields the needle, helping to protect from needlestick injury and associated risk.‡ Controls blood leakage, eliminating the need to use your hand for venous compression during insertion, repeated connections and blood draws†,‡
-
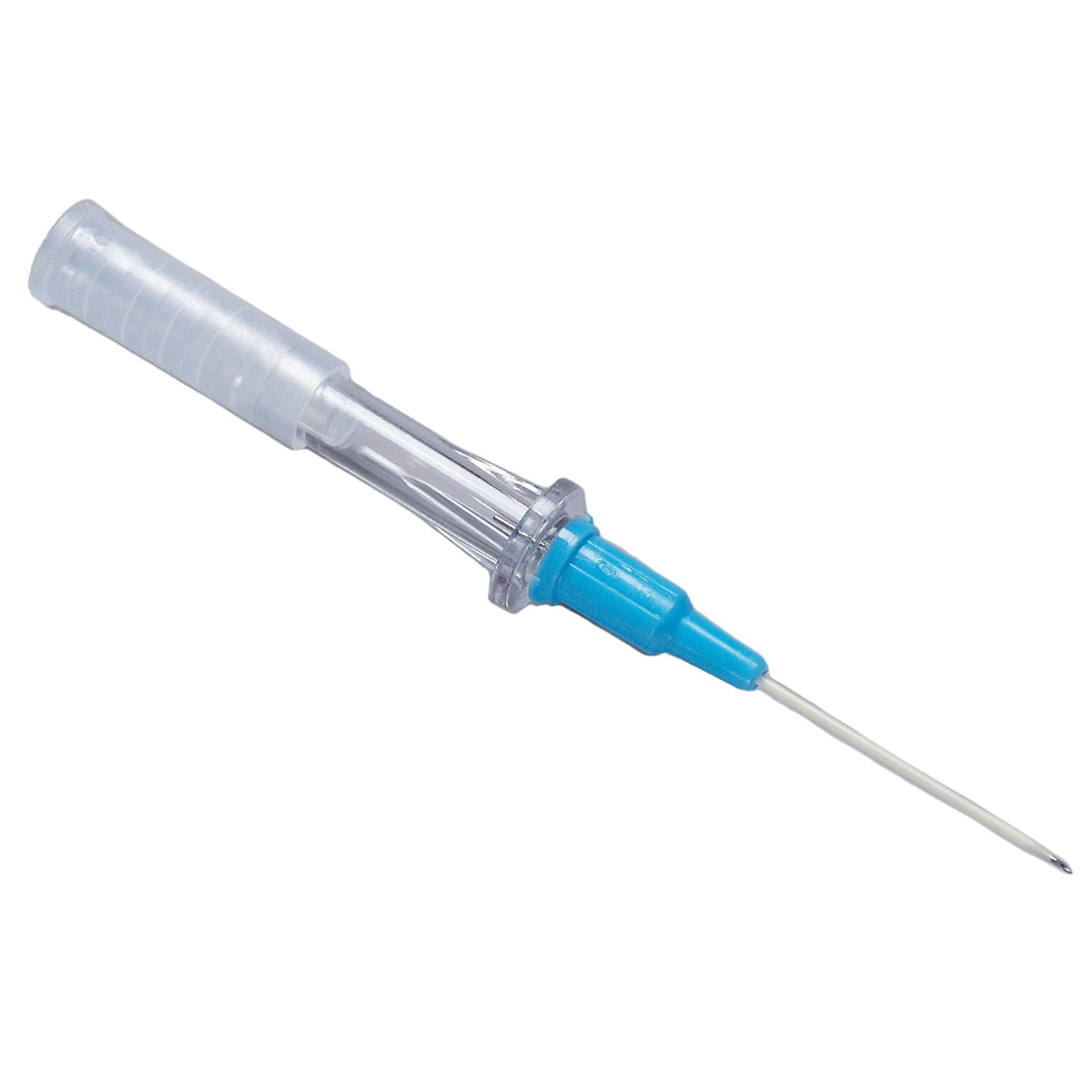
The Angiocath™ IV Catheter offers a wide range of products designed to provide peripheral vascular access to help meet clinicians’ needs. The catheters are made with FEP material with a clear flash chamber to help make blood flashback easily identifiable.
-
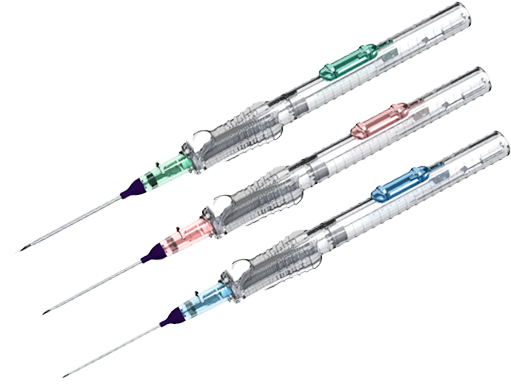
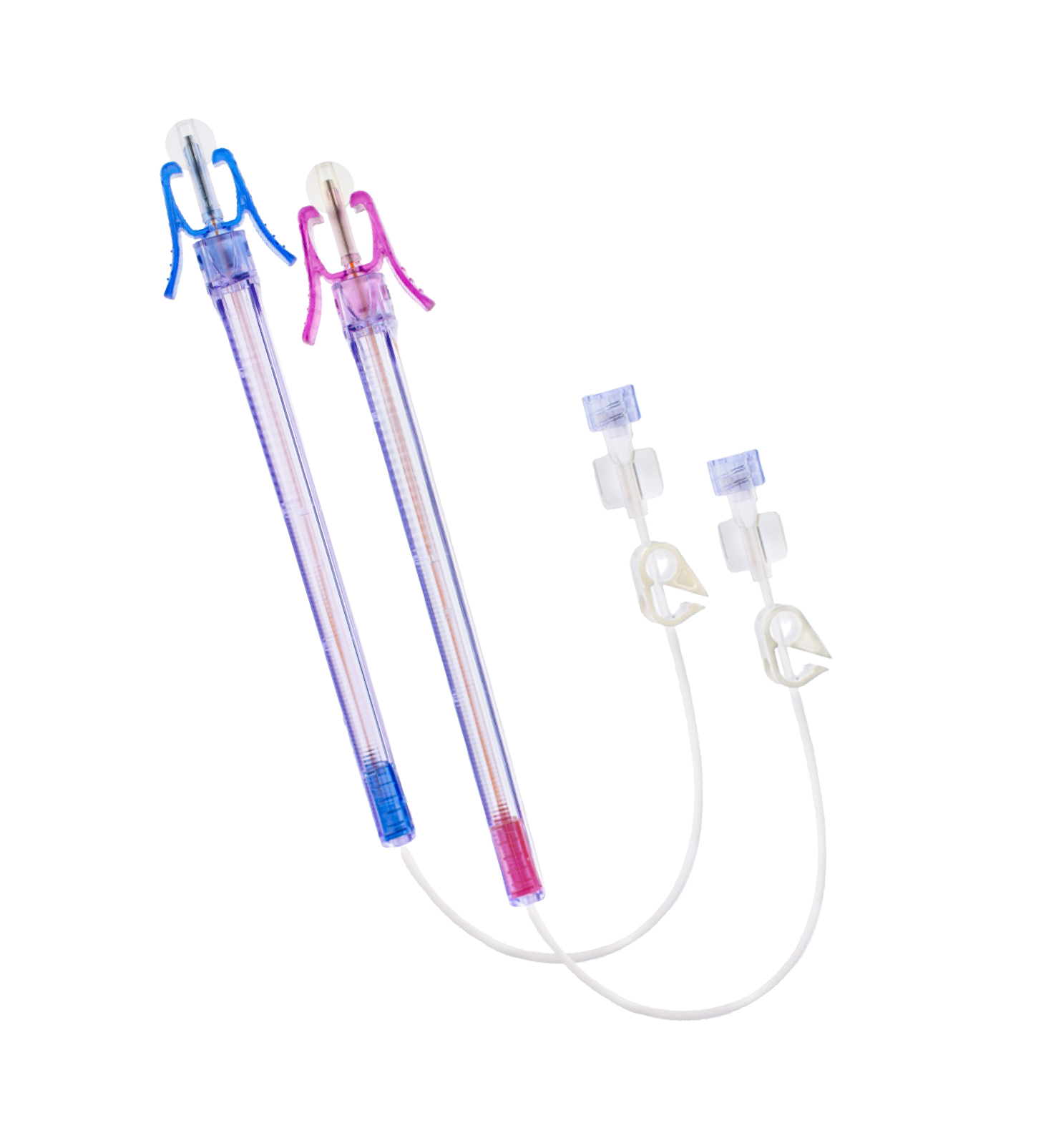
The AccuCath Ace™ Intravascular Catheter system consists of a radiopaque catheter with a valve mechanism delivered over a guidewire with an atraumatic tip design; a flashback chamber to enhance flashback visualization, and a safety container that prevents sharp injuries.
*BD Nexiva Catheter System with 3M Tegaderm IV Securement Dressing compared to B. Braun Introcan Safety catheter with Statlock IV Ultra stabilization device and non-bordered dressing
**22 G–24 G
†Up to 10 seconds
‡Compared to a non-safety, non-blood control catheter
Chart detailing the features of the BD PIVC products.
| Blood | Safety | BD® Instaflash™ Technology | Catheter material | Configuration | Offered with BD® MaxZero™
Needle-free Connector |
| BD® Nexiva™ Catheter System with NearPort™ IV Access | Yes | Passive | Yes | Proprietary BD® Vialon™ Catheter Material | Intregrated catheter with dual port | Yes |
| BD® Nexiva™ Catheter System | Yes | Passive | Yes | Proprietary BD® Vialon™ Catheter Material | Integrated catheter with single and dual port option | Yes |
| BD® Nexiva Diffusics™ Catheter System | Yes | Passive | Yes | Proprietary BD® Vialon™ Catheter Material | Integrated catheter with single port | Yes |
| BD® Insyte Autoguard™ Shielded IV Catheter | YesΔ | Active | Yes | Proprietary BD® Vialon™ Catheter Material | Straight catheter with winged option | No |
| BD® Cathena™ Safety IV Catheter with BD Multiguard™ Technology | YesΔ | Passive | Yes | Proprietary BD® Vialon™ Catheter Material | Straight catheter with winged option | No |
| BD® Angiocath™ IV Catheter | No | No | Yes | PTFE | Straight catheter | No |
| AccuCath Ace™ IV Catheter | Yes | Active | Yes | Proprietary BD® Vialon™ Catheter Material | Straight catheter with guidewire | No |
ΔWith non blood control option
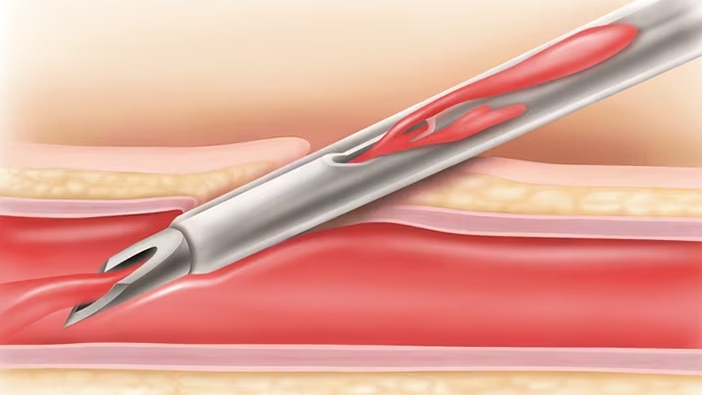
BD® Instaflash™ Needle Technology
Incorporates a notched needle which is designed to provide immediate confirmation of vessel entry, and clinically demonstrated to improve first-attempt insertion success, reducing painful hit-and-miss insertions.§
BD® Vialon™ Catheter Material
Multiple clinical studies over 30 years have demonstrated that IV catheters made of BD Vialon™ Catheter Material were easy to insert, have lower phlebitis rates and provide longer dwell timesꞵ
§Compared to a non-notched needle
ꞵCompared to an FEP catheter
Foundational knowledge. Practical
guidance. Expert-led training.
Vascular access complications are common—but often preventable.
As part of our commitment to vascular access excellence, we offer educational resources designed to advance practice at each step: from selection and insertion to care and maintenance. Whether you’re building foundational skills or refining advanced techniques, our flexible learning options are developed to support all experience levels—and help promote safe, consistent vascular access practices.
Flexible learning designed to meet your team’s needs
Instructor-led clinical education
Led by a BD Clinical Specialist, this 60–90-minute interactive session covers insertion and beyond—focusing on real-world scenarios and best practices across the full PIVC care pathway.
View and download the BD® PIVC Excellence Toolkit:
On-demand eLearning
These self-paced modules offer accessible training on topics such as PIVC insertion, flushing and locking fundamentals, care and maintenance best practices and more.
Visit the BD® Learning Academy:
Webinars
Expert-led sessions that deliver evidence-based insights on topics such as complication reduction, catheter occlusion prevention and safety in vascular access.
View our on-demand webinars: